Preparation method of phenanthridine heterocyclic compounds
The technology of a compound, phenanthridine, is applied in the field of preparation of phenanthridine heterocyclic compounds, which can solve the problems of unfriendly bromide, poor functional group tolerance, environmental protection and greenness, etc., and achieve easy control and applicable scope of substrates Wide, important commercial value and the effect of industrial application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
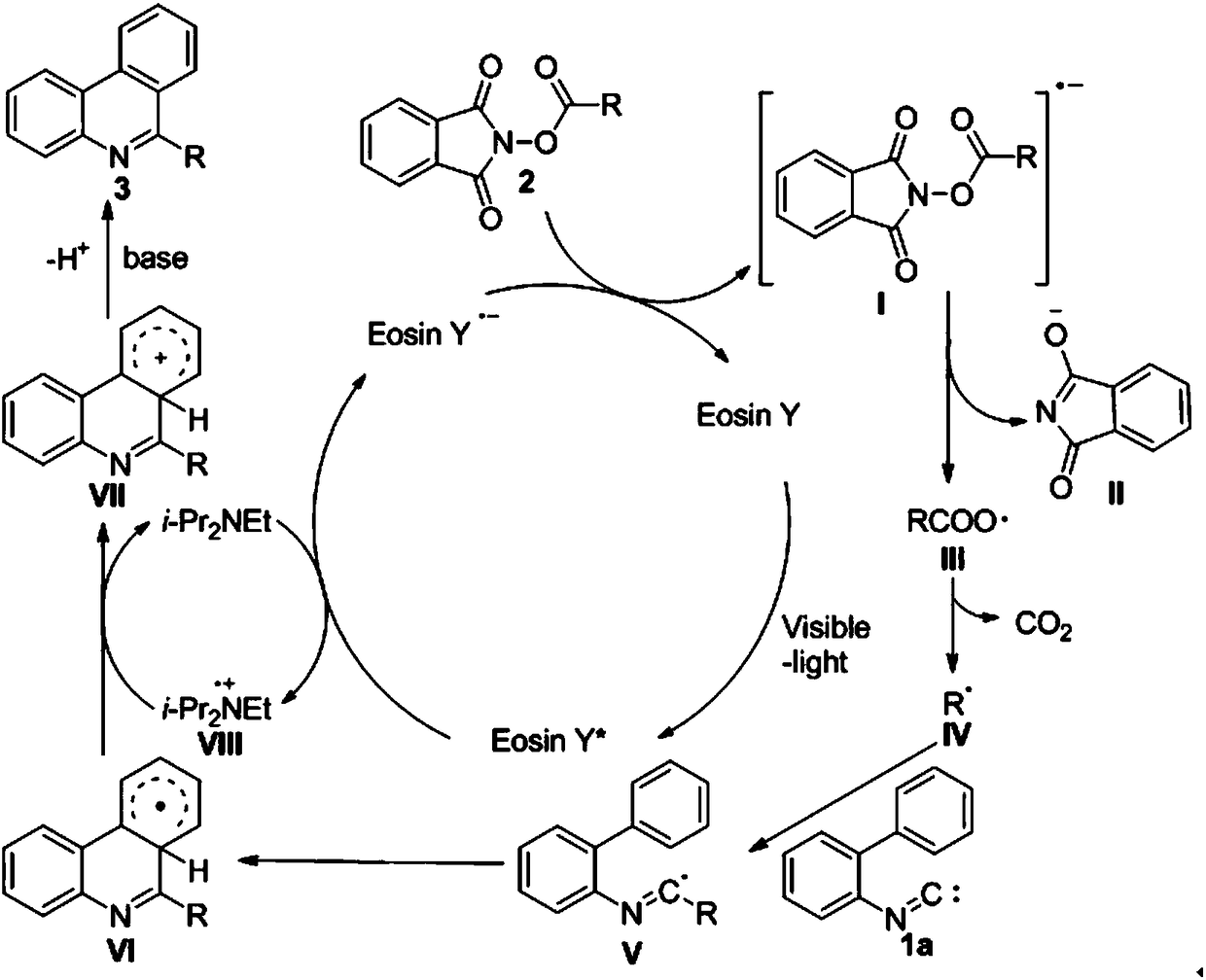
[0034] Isonitrile 1a (0.30mmol), N-(acyloxy)phthalimide 2a (0.60mmol), Eosin Y (5mol%), sodium bicarbonate (0.36mmol), N,N-di Isopropylethylamine (0.60 mmol) was dissolved in dimethyl sulfoxide (3.0 mL), and placed under a 18W white light for 24 hours. After the reaction was completed, the reaction system was diluted with water, the aqueous phase was extracted three times with ethyl acetate, the organic layers were combined, dried with anhydrous sodium sulfate, and the solvent was removed by rotary evaporation on a water pump under reduced pressure to obtain a crude product, which was passed through a chromatographic silica gel column. Purification (ethyl acetate:petroleum ether=1:40) gave 48.1 mg of yellow solid product 3aa with a yield of 83%.
[0035] Data representation: 1 H NMR (400MHz, CDCl 3 )δ8.61(d, J=8.4Hz, 1H), 8.53(d, J=8.0Hz, 1H), 8.21(d, J=8.0Hz, 1H), 8.10(d, J=8.0Hz, 1H) ,7.83(t,J=7.6Hz,1H),7.70(q,J=8.1Hz,2H),7.61(t,J=7.5Hz,1H),3.04(s,3H); 13 C NMR (100MHz, ...
Embodiment 2
[0037] Isocyanide 1a (0.30mmol), N-(acyloxy)phthalimide 2b (0.60mmol), eosin Y (5mol%), sodium bicarbonate (0.36mmol), N,N-di Isopropylethylamine (0.60 mmol) was dissolved in dimethyl sulfoxide (3.0 mL), and placed under a 18W white light for 24 hours. After the reaction was completed, the reaction system was diluted with water, the aqueous phase was extracted three times with ethyl acetate, the organic layers were combined, dried with anhydrous sodium sulfate, and the solvent was removed by rotary evaporation on a water pump under reduced pressure to obtain a crude product, which was passed through a chromatographic silica gel column. (Ethyl acetate:petroleum ether=1:40) purified to obtain 53.5 mg of white solid product 3ab, with a yield of 86%.
[0038] Data representation: 1 H NMR (400MHz, CDCl 3)δ8.62(d, J=8.3Hz, 1H), 8.52(d, J=8.2Hz, 1H), 8.25(d, J=8.2Hz, 1H), 8.14(d, J=8.1Hz, 1H) ,7.81(t,J=7.6Hz,1H),7.69(dt,J=8.0,7.2Hz,2H),7.61(t,J=7.5Hz,1H),3.41(q,J=7.6Hz,2H) ,1.51(...
Embodiment 3
[0040] Isonitrile 1a (0.30mmol), N-(acyloxy)phthalimide 2c (0.60mmol), Eosin Y (5mol%), sodium bicarbonate (0.36mmol), N,N-di Isopropylethylamine (0.60 mmol) was dissolved in dimethyl sulfoxide (3.0 mL), and placed under a 18W white light for 24 hours. After the reaction was completed, the reaction system was diluted with water, the aqueous phase was extracted three times with ethyl acetate, the organic layers were combined, dried with anhydrous sodium sulfate, and the solvent was removed by rotary evaporation on a water pump under reduced pressure to obtain a crude product, which was passed through a chromatographic silica gel column. Purification (ethyl acetate:petroleum ether=1:40) gave 98.2 mg of white solid product 3ac with a yield of 86%.
[0041] Data representation: 1 H NMR (400MHz, CDCl 3 )δ8.61(d, J=8.0Hz, 1H), 8.51(d, J=8.0Hz, 1H), 8.23(d, J=8.0Hz, 1H), 8.14(d, J=8.0Hz, 1H) ,7.80(t,J=7.6Hz,1H),7.69(dt,J=15.2,7.4Hz,2H), 7.60(t,J=7.5Hz,1H),3.44–3.30(m,2H),1.92( dt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com