Preparation method of 6-H-phenanthridine compounds by one-pot process
A technology for 6-H-compounds, applied in organic chemistry, bulk chemical production, etc., can solve the problems of few reports on the synthesis of phenanthridine compounds, and achieve the effects of easy reaction, high yield and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0023] The embodiments of the present invention will be further described below in conjunction with the accompanying drawings and specific examples, but the present invention is not limited to the following embodiments, and various changes may be made within the scope of knowledge of those skilled in the art.
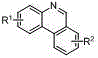
[0024] A "one-pot method" for the preparation of 6- H -The method of phenanthridine compound, its reaction process is as follows:
[0025]
[0026] Among them, PG (ProtectedGroup) is a protecting group, including -Ms (methylsulfonyl), -Ts (p-toluenesulfonyl), -Ns (p-nitrobenzenesulfonyl) and -Ac (acetyl); substituent R 1 and R 2 Any of alkyl, alkoxy, acyl, nitro, cyano, and halogen.
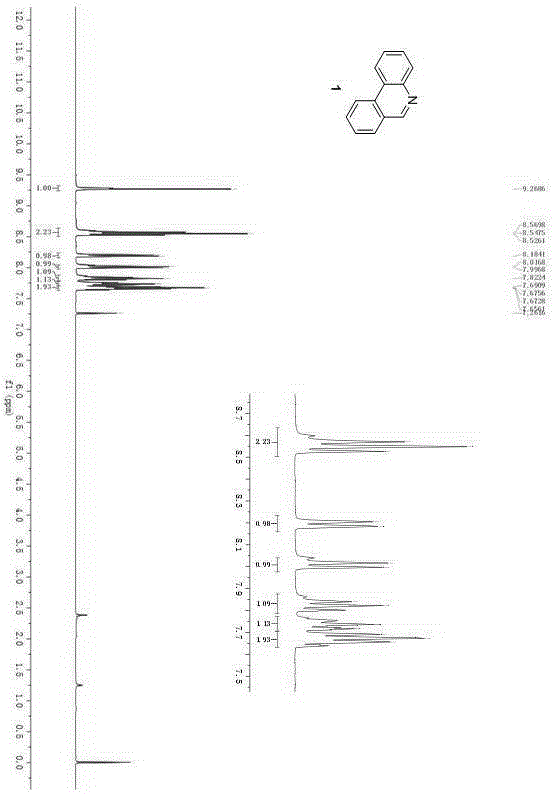
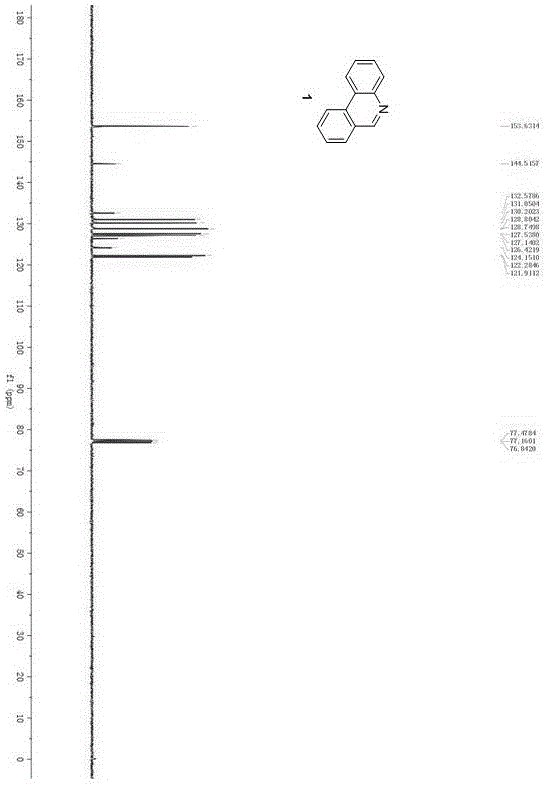
[0027] The operation steps of synthetic representative compound 1:
[0028]
[0029] Will N -Protected arylamine, o-bromobromobenzyl compound, metal palladium catalyst, ligand and base are added in an organic solvent, reacted under heating and inert gas protection conditions, an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com