Intermediate compound of Lefamulin and application of intermediate compound in preparation of Lefamulin
A compound and intermediate technology, applied in the preparation of organic compounds, carbamic acid derivatives, thiols, etc., can solve the problems of low yield and high cost of Lefamulin preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
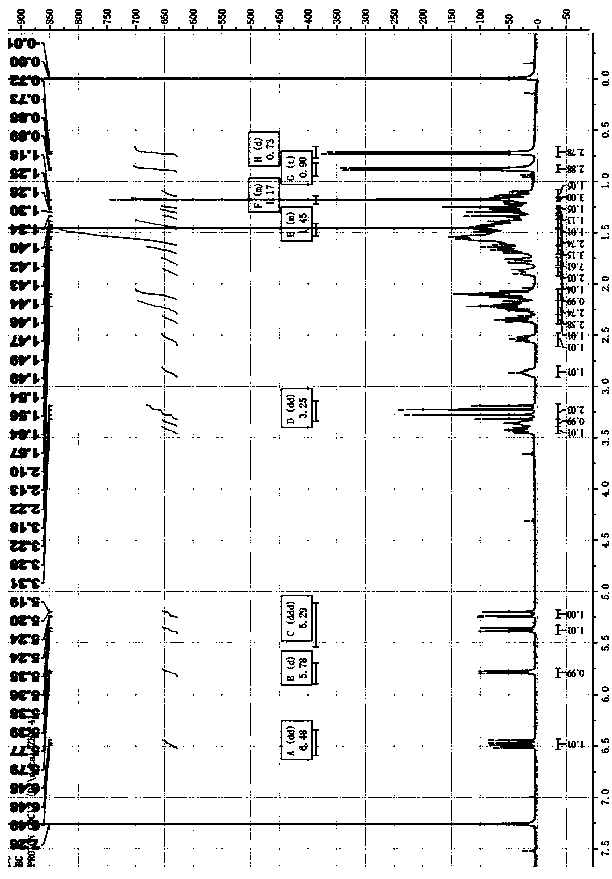
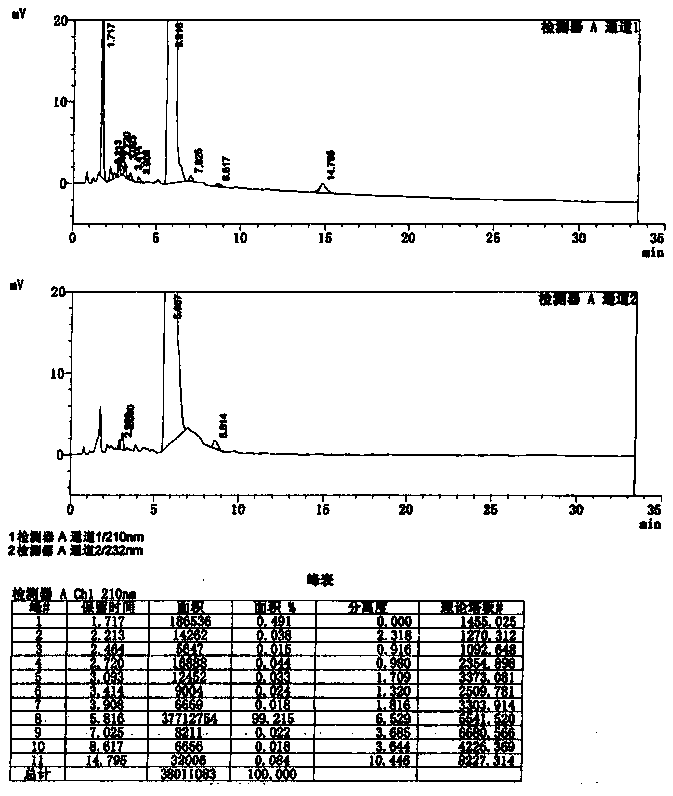
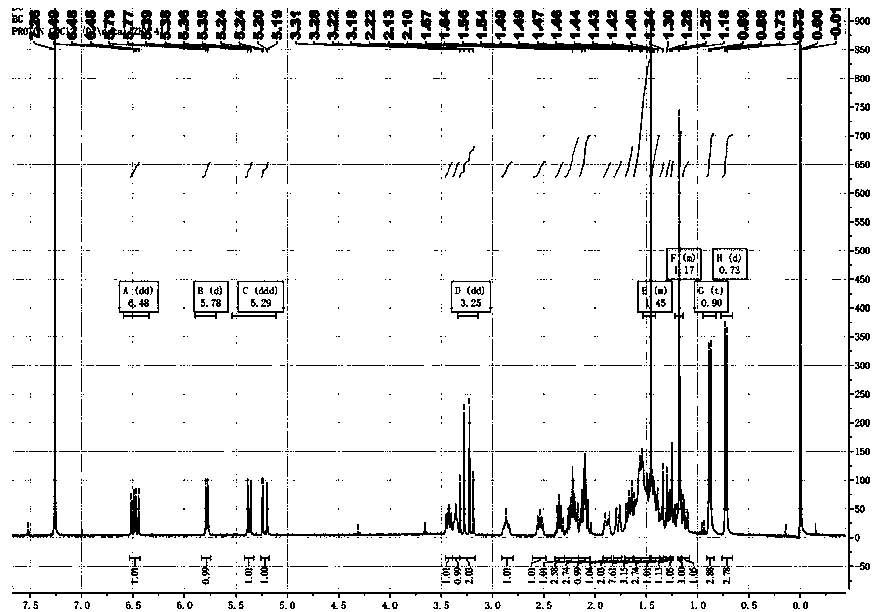
Image
Examples
Embodiment 1
[0074] Embodiment 1: the preparation method of compound Lefamulin
[0075] Using commercially available (1R,3R,4R)-4-bromo-3-hydroxy-cyclohexylcarboxylic acid as the starting material to prepare Lefamulin, the process is as follows:
[0076] ;
[0077] details as follows:
[0078] (1) Preparation of ((1R,3R,4R)-4-bromo-3-hydroxy-cyclohexyl) tert-butoxycarbonylamide
[0079] Add 4.8kg of (1R,3R,4R)-4-bromo-3-hydroxycyclohexylcarboxylic acid (commercially available, see Organic Process Research and Development, 2019, vol. 23, # 4, p. 524-534), toluene 50L, add triethylamine 1.3eq under stirring condition, then add diphenylphosphoryl azide 1.1eq, then heat to reflux, when the reaction is finished, cool down to 80°C, dropwise add tert-butanol 5L, Then add catalyst cuprous halide 5g, continue to react for 2 hours. Purified to obtain 3.1 kg of the target compound ((1R,3R,4R)-4-bromo-3-hydroxy-cyclohexyl) tert-butoxycarbonyl amide (formula A).
[0080] Similarly, benzyloxycarb...
Embodiment 2
[0091] Embodiment 2: the preparation method of compound Lefamulin
[0092] Adopting commercially available commercially available (1R,3R,4R)-4-bromo-3-hydroxy-cyclohexyl formic acid to prepare Lefamulin, the process is:
[0093] ;
[0094] details as follows:
[0095] (1) Preparation of (1R,3R,4R)-4-bromo-3-((tert-butyldimethylsilyl)oxy)cyclohexylcarboxylic acid
[0096] Add 60L of dichloromethane and 4.8kg of (1R,3R,4R)-4-bromo-3-hydroxycyclohexylcarboxylic acid (commercially available, see Organic Process Research and Development, 2019, vol. 23, # 4, p.524-534) and 1.2eq of tert-butyldimethylchlorosilane, add 1.3eq of triethylamine dropwise under stirring condition, stir the reaction to the end after adding, wash the reaction system with water, concentrate dichloromethane, 6.4 kg of the product (1R,3R,4R)-4-bromo-3-((tert-butyldimethylsilyl)oxy)cyclohexylcarboxylate (formula 1) was obtained. The yield is 98%.
[0097] Similarly, reacting with trimethylchlorosilane, tr...
Embodiment 3
[0109] Embodiment 3: the preparation method of compound Lefamulin
[0110] Adopting commercially available commercially available (1R,3R,4R)-4-bromo-3-hydroxy-cyclohexyl formic acid to prepare Lefamulin, the process is:
[0111] ;
[0112] (1) Preparation of (1R,3R,4R)-4-bromo-3-(benzyloxy)cyclohexylcarboxylic acid
[0113] Add 100 ml of DMF, 30 g of (1R,3R,4R)-4-bromo-3-hydroxycyclohexylcarboxylic acid, 1.2 eq of benzyl bromide, and 1.3 eq of sodium hydrogen into a 250 ml three-necked flask. The reaction system was quenched with water and extracted with dichloromethane to obtain 21.4 g of the target compound (ie Formula 1).
[0114] Similarly, with p-methoxybenzyl chloride, methoxychloromethyl ether or methoxybromomethyl ether, 3,4-dihydro-2H-pyran, 2-(trimethylsilyl)ethoxymethyl Chlorine, allyl chloride, 3,4-dihydro-2H-pyran, 2-(trimethylsilyl) ethoxymethyl chloride, allyl chloride and other reactions can obtain other alkanes of formula 1' Ether protected analogues. W...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com