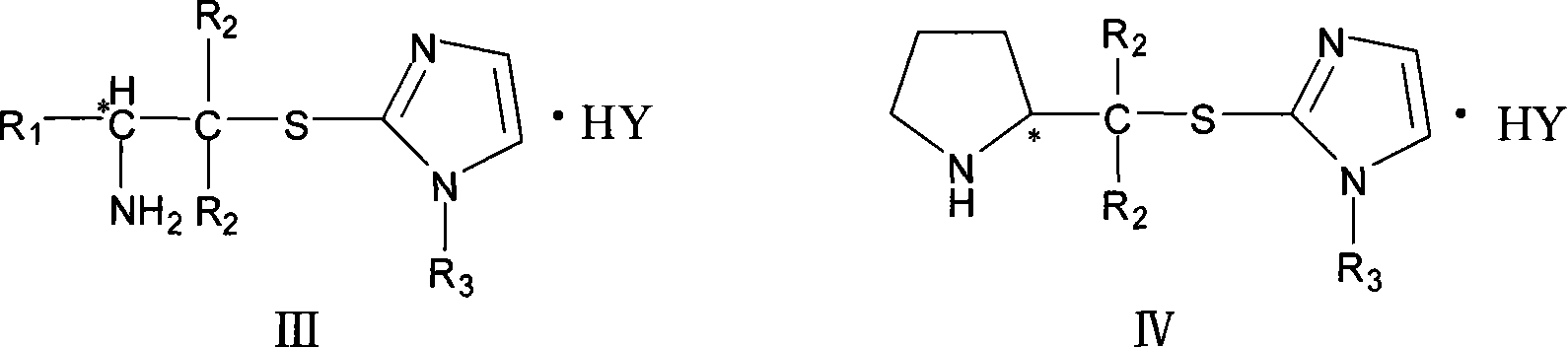
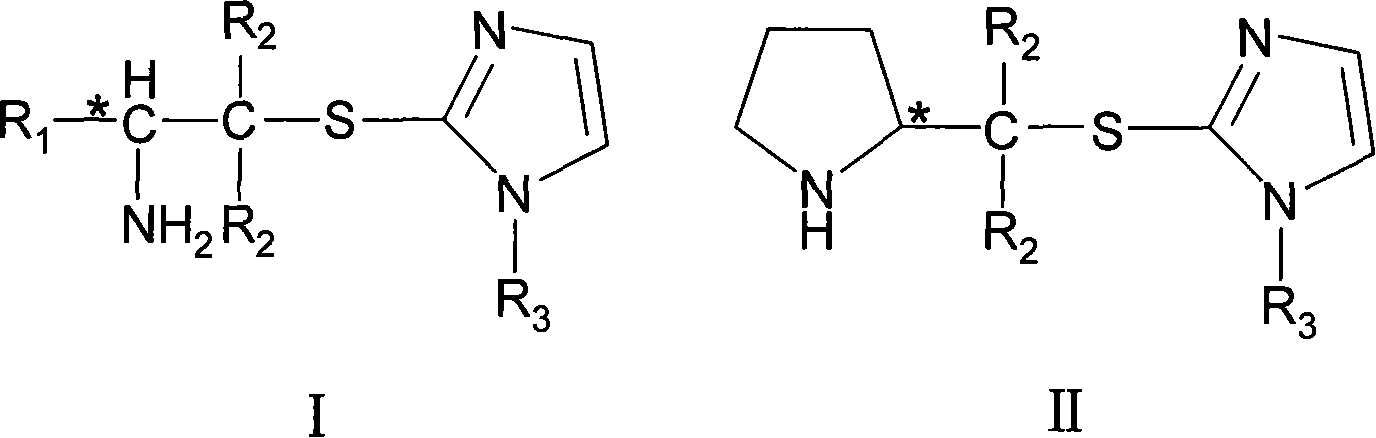
Chirality amine protonic acid salt containing imidazole sulfur ether structure and preparation method and usage thereof
A technology of protonic acid salt and chiral amine, which is applied in the field of chiral amine protic acid salt to achieve good chiral induction properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Example 1: Preparation of 2-((S)-2-aminopropylthio)-1-hexylimidazole
[0061] Add (S)-1-(bromomethyl)ethylamine hydrobromide (21.90g, 0.1mol), 2-mercapto-1-hexylimidazole (18.41g, 98%, 0.1mol) and ethanol in a 100mL three-necked flask (60mL), reflux reaction for 12h, after neutralization, desolvation under reduced pressure, washed with ethyl acetate (2 × 20mL), after distillation to remove the solvent, column chromatography separation and purification to obtain the target compound (22.85g, yield 95% ), its specific rotation [α] D 20 =+32.1°.
Embodiment 2
[0062] Example 2: Preparation of 2-((S)-2-aminopropylthio)-1-methylimidazole
[0063] (S)-1-(bromomethyl)ethylamine hydrobromide (21.90g, 0.1mol), 2-mercapto-1-methylimidazole (18.41g, 98%, 0.1mol) and 1,2-dichloroethane (60mL), reflux reaction for 12h, after neutralization, desolvation under reduced pressure, washed with ethyl acetate (2 × 20mL), and then distilled to remove the solvent to obtain the target compound (16.20g, yield 95%), its specific rotation [α] D 20 =+33.2°.
Embodiment 3
[0064] Example 3: Preparation of 2-((S)-2-aminobutylthio)-1-ethylimidazole
[0065] Add (S)-1-(chloromethyl)propylamine hydrochloride (14.4g, 0.1mol), 2-mercapto-1-ethylimidazole (12.82g, 98%, 0.1mol) and acetonitrile ( 10mL), reflux reaction for 12h, after neutralization, desolventization under reduced pressure, washing with ethyl acetate (2 × 20mL), and distillation to remove the solvent, separation and purification by column chromatography to obtain the target compound (14.95g, yield 75%) , its specific rotation [α] D 20 =+33.5°.
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific rotation | aaaaa | aaaaa |
| specific rotation | aaaaa | aaaaa |
| specific rotation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com