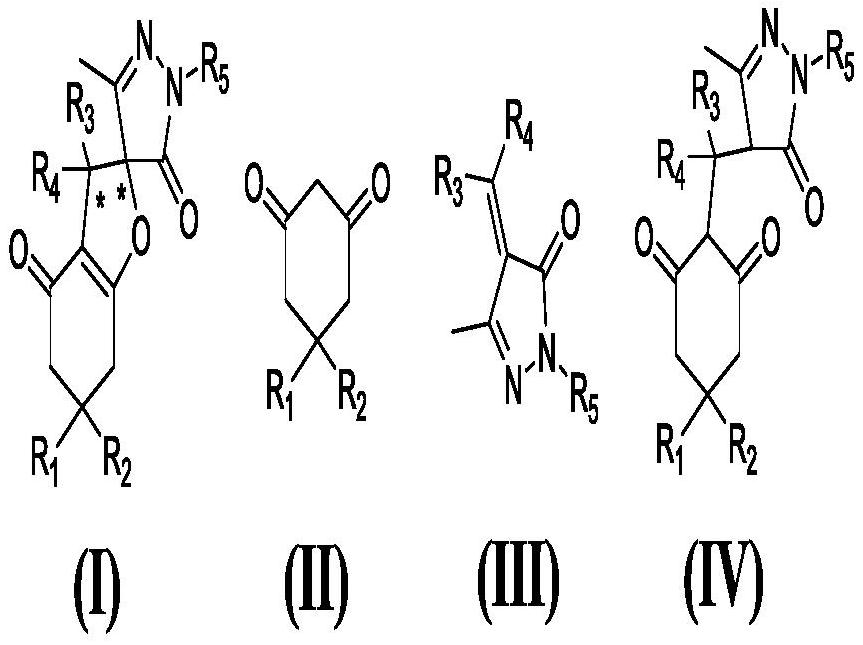
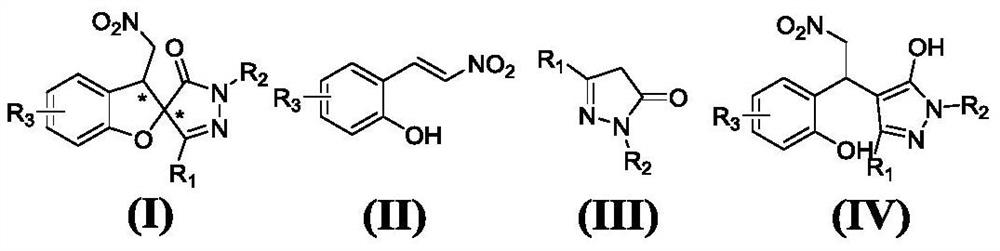
A kind of iodine medium preparation method of chiral pyrazole spirofuran compound
A technology for pyrazole spirofuran and compounds, which is applied in the field of iodine medium preparation of chiral pyrazole spirofuran compounds, can solve the problems of increasing post-processing costs, etc., and achieve good reaction characteristics, mild reaction conditions, and good selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0077] Example 1: (2R,3S)-3'-methyl-3-nitromethyl-1'-phenyl-3H-spiro[benzofuran-2,4'-pyrazole]-5'(1 'H)-ketone;
[0078]
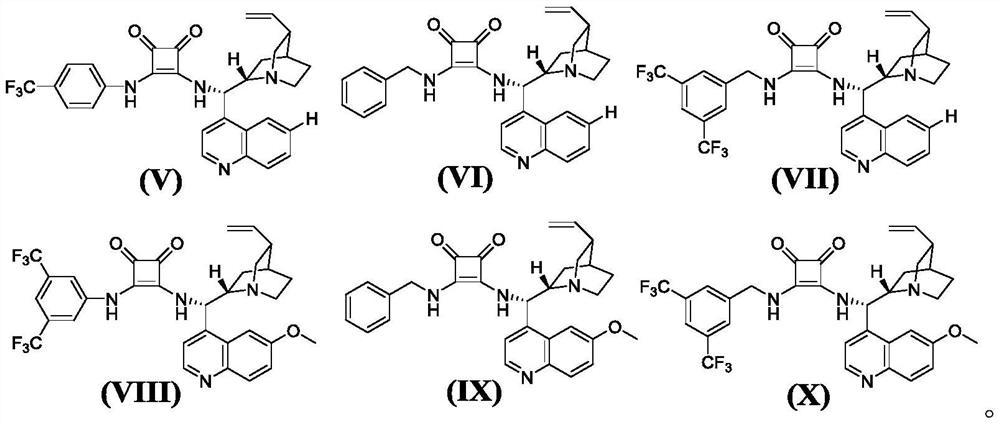
[0079] (A) Take 10mL clean small test tube, add o-hydroxynitroalkene (0.2mmol, 0.033g), 5-methyl-2-phenyl-pyrazolone (0.2mmol, 0.0348g), chiral squaraine catalyst V (0.002mmol, 0.0011g), solvent dichloromethane (1mL), after reacting at 25°C for 6h, a mixture containing intermediate compound 1-A was obtained;
[0080] (B) Remove the solvent from the mixture containing intermediate compound 1-A, add cuprous iodide (0.076g, 0.4mmol), lithium carbonate (0.4mmol, 0.0296g), solvent dichloromethane (1mL), 25°C After reacting for 8 hours, extract with ethyl acetate (3×10mL), and desolvate the organic phase under reduced pressure, use ethyl acetate:petroleum ether=1:10 mixed solvent as eluent; 200-300 mesh column chromatography silica gel as filler , the target product (0.0593g, 88%yield, 57%ee,>99:1dr) obtained by column chromatography separation and purifica...
Embodiment 2
[0081] Example 2: (2R,3S)-3'-ethyl-3-nitromethyl-1'-phenyl-3H-spiro[benzofuran-2,4'-pyrazole]-5'(1 'H)-ketone;
[0082]
[0083] (A) Take 10mL clean small test tube, add o-hydroxynitroalkene (0.2mmol, 0.033g), 5-ethyl-2-phenyl-pyrazolone (0.2mmol, 0.0376g), chiral squaraine catalyst VI (0.02mmol, 0.0096g), solvent toluene (2mL), after reaction at 0°C for 24h, a mixture containing intermediate compound 2-A was obtained;
[0084] (B) Remove the solvent from the mixture containing intermediate compound 2-A, then add iodobenzene acetate (0.8mmol, 0.2576g), sodium carbonate (0.2mmol, 0.0212g), solvent toluene (2mL), and react at 25°C for 1h Finally, extract with ethyl acetate (3 × 10mL), the organic phase is desolvated under reduced pressure, use ethyl acetate:petroleum ether=1:10 mixed solvent as eluent; 200-300 mesh column chromatography silica gel is as filler, column The target product obtained by chromatographic separation and purification (0.0604g, 86%yield, 78%ee, >99:1...
Embodiment 3
[0085] Example 3: (2R,3S)-3-nitromethyl-1',3'-diphenyl-3H-spiro[benzofuran-2,4'-pyrazole]-5'(1'H )-ketone;
[0086]
[0087] (A) Take 10mL clean small test tube, add o-hydroxynitroalkene (0.2mmol, 0.033g), 2,5-diphenyl-pyrazolone (0.2mmol, 0.0472g), chiral squaraine catalyst VII ( 0.04mmol, 0.0246g), solvent ethyl acetate (3mL), after reaction at -20°C for 20h, a mixture containing intermediate compound 3-A was obtained;
[0088] (B) Remove the solvent from the mixture containing intermediate compound 3-A, then add hydrogen iodide (2mmol, 0.256g), potassium carbonate (0.1mmol, 0.0614g), solvent ethyl acetate (3mL), and react at 25°C After 2h, extract with ethyl acetate (3×10mL), and desolvate the organic phase under reduced pressure, use ethyl acetate:petroleum ether=1:10 mixed solvent as eluent; 200-300 mesh column chromatography silica gel as filler, The target product (0.0599g, 75%yield, 84%ee, >99:1dr) obtained by column chromatography separation and purification, 1 H ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com