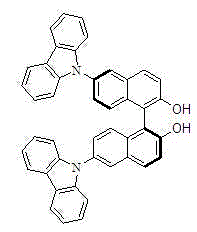
Chiral 6, 6'-2 carbazole base binaphthol
A technology of carbazolyl binaphthol and brominated binaphthol is applied in the field of chiral 6,6'-dicarbazolyl binaphthol, which can solve the problems of optical rotation characterization, lack of relevant characterization data, and non-existence.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0011] Add R configuration 6,6'-dibromobinaphthol (1.0 mmol), carbazole (1.0 mmol), tris(dibenzylideneacetone) dipalladium (18.3 mg , 0.02 mmol), 2-(di-tert-butylphosphine) biphenyl (8.95 mg, 0.03 mmol), potassium carbonate (414 mg, 3.0 mmol). The ground-mouth test tube was sealed with an inversion rubber stopper, then replaced with high-purity argon for 3 times, and then injected with 5 ml of toluene through the syringe. Put the sealed ground-mouth test tube in a preheated oil bath at 100°C, and stir for 24 hours to react. The test tube was cooled to room temperature, and the acidity of the reaction mixture was carefully adjusted to pH 5-6 with 2 mol / L dilute hydrochloric acid. After quenching, the reaction mixture was extracted three times with ethyl acetate, and the combined organic phases were dried over anhydrous magnesium sulfate, filtered, and concentrated under reduced pressure. The obtained crude product was separated and purified by silica gel column chromatograph...
Embodiment 2
[0013] Use S configuration 6,6'-dibromobinaphthol instead of R configuration 6,6'-dibromobinaphthol in Example 1 to obtain S configuration 6,6'-dicarbazolyl binaphthol . Yield: 386.1 mg, yield: 63%, yellow solid. Mp. 193-195 ℃; [α] D 25 = -108.1 (c = 0.45, THF).
Embodiment 3
[0015] Use racemic 6,6'-dibromo-binaphthol to replace the R-configuration 6,6'-dibromo-binaphthol in Example 1 to obtain racemic 6,6'-dicarbazolyl-binaphthol . Yield: 376.5 mg, yield: 61%, yellow solid. Mp. 207-209℃.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com