Azobenzene polymer as well as preparation method and application thereof
A technology of azobenzene and polymers, applied in the field of supramolecular chiral immobilization, can solve the problems of high price, limited types of chiral polymers, and constraints on the development of chiral polymers, and achieve the effect of avoiding synthesis steps and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
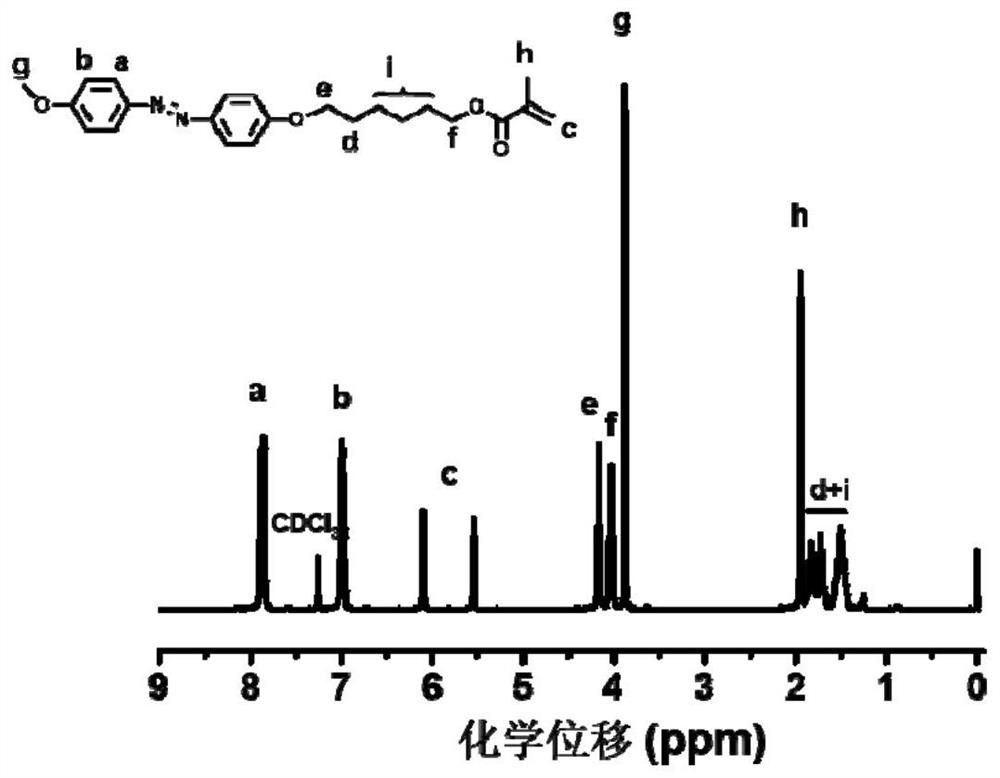
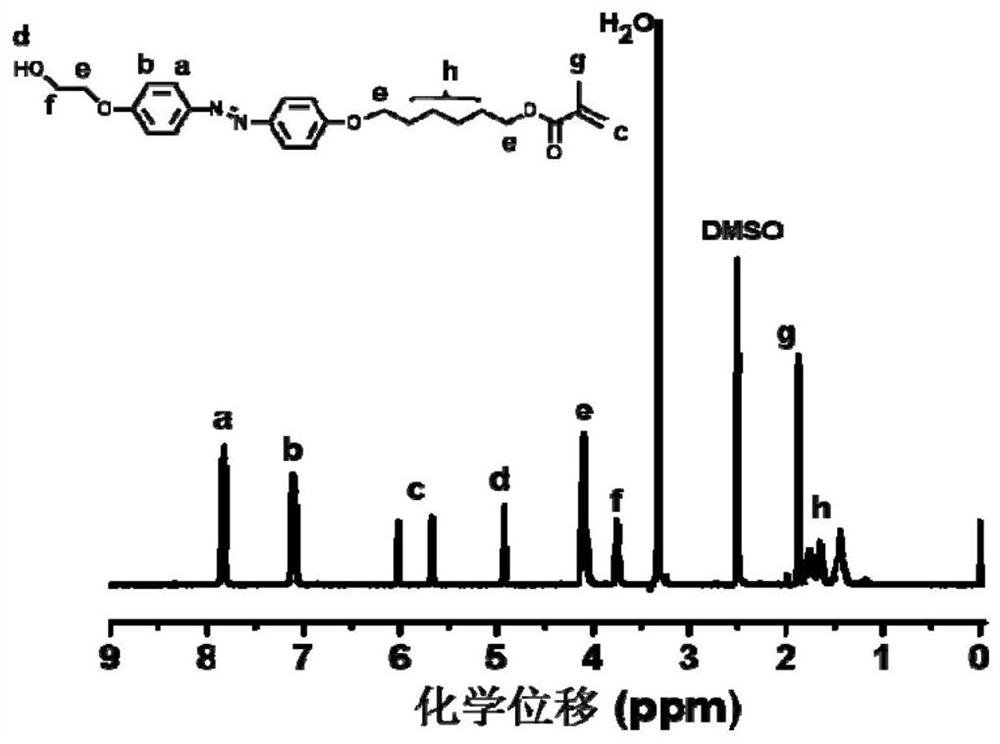
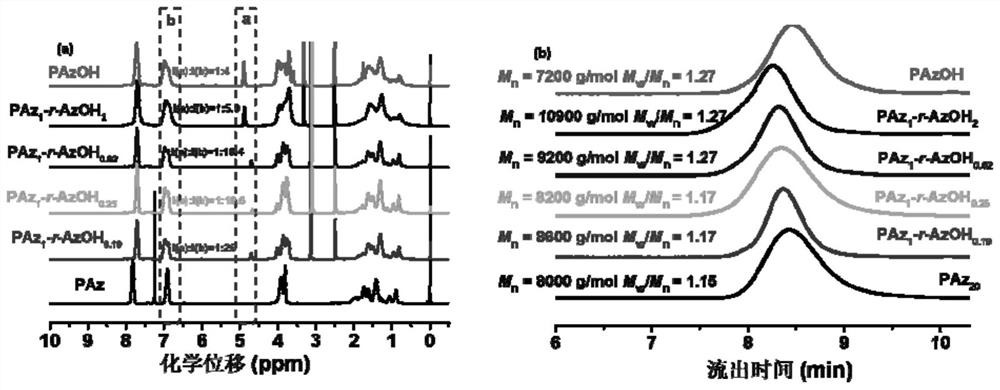
[0106] 1) Synthesis of achiral methoxyazobenzene monomer
[0107] The raw material methoxyaniline (12.32 g, 0.1 mol) and concentrated hydrochloric acid were added to deionized aqueous solution (80 mL), magnetically stirred in an ice-salt bath for 0.5 h, and then 30 mL NaNO 2 (7 g, 0.1 mol / L) aqueous solution, after the dropwise addition, the reaction was continued for 0.5 h in an ice-salt bath to obtain a diazonium salt solution, and the system was red.
[0108] Phenol (16 g, 0.15 mol), NaOH (8 g, 0.2 mol), NaHCO 3 (8.4 g, 0.1 mol) was dissolved in 50 mL of deionized water, under ice-salt bath conditions (control temperature 0-5 °C), mechanically stirred, and then the previously obtained diazonium salt solution of p-methoxyaniline was added dropwise to the above In the phenol solution, the ice-salt bath condition still needs to be maintained at this time, and the solution gradually changes from colorless to yellow, and finally turns into brownish yellow. After the dropwise a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com