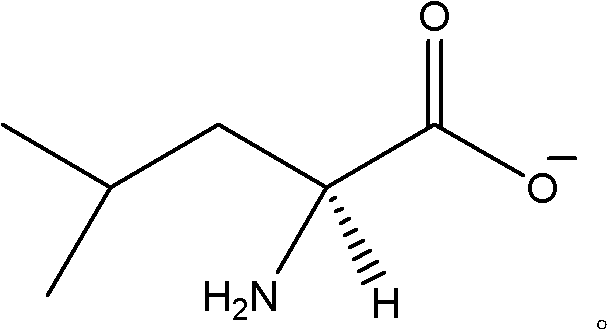
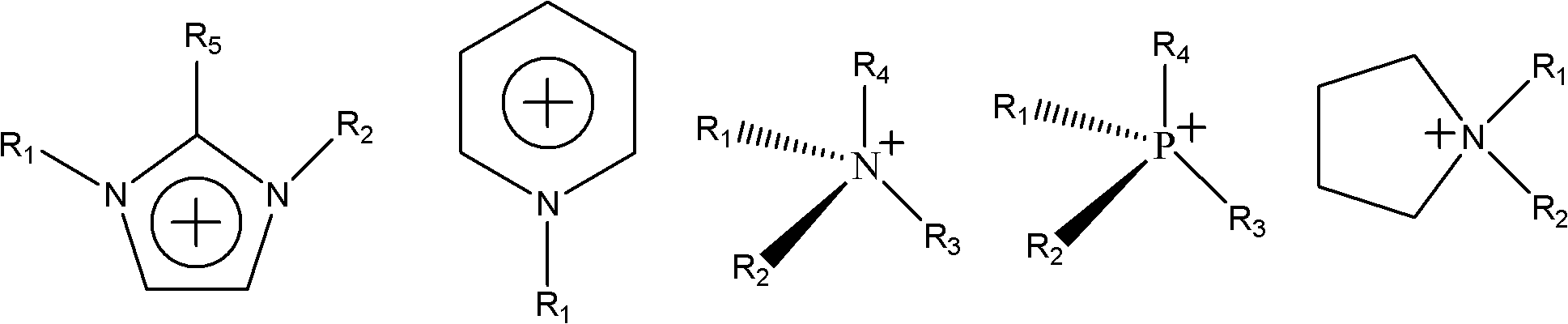
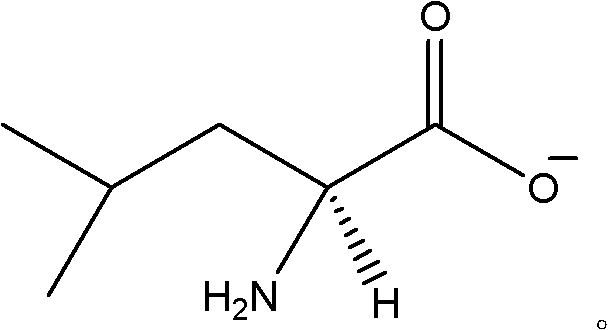
Chiral leucine ion liquid
A leucinate and ionic liquid technology, applied in the field of chemical materials, can solve the problems of limited types of chiral ionic liquids, and achieve the effects of low cost, easy realization, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1: Synthesis of L-leucine-1-methyl-3-butylimidazolium ionic liquid
[0027] Weigh 50g of chloro-1-methyl-3-butylimidazole and put it into a three-necked flask, add 150ml of acetone solution, 43.84g of L-sodium leucine in turn, and add the rotor, and nitrogen is passed through one inlet, The other outlet is plugged with a drying tube, and the nitrogen flow rate is controlled to be one bubble per second; the three-necked bottle is stirred on a magnetic stirrer, and stirred for 3 days;
[0028] After the reaction was completed, a centrifuge was used to remove insoluble NaCl to obtain 160 ml of oily liquid. Then add 1.5g activated Al 2 o 3 , and added to the rotor, stirred on a magnetic stirrer for 30min, filtered to remove Al 2 o 3 ;
[0029] Remove Al 2 o 3 Afterwards, quickly remove acetone with rotary evaporation, control the temperature at 48-52°C to obtain an oily liquid, put it in a vacuum oven, control the temperature at 120°C to remove residual ac...
Embodiment 2
[0030] Embodiment 2: The synthesis of L-leucine-1-methyl-3-butylimidazole ionic liquid, compared with Example 1, the difference is that chloro-1-methyl-3-butylimidazole uses The following synthesis not only saves costs, but also increases the yield of the final ionic liquid, specifically:
[0031] Add 100ml of N-methylimidazole and 150ml of n-butane chloride into a 500ml three-necked flask, and heat to reflux under the condition of a silicone oil bath: first, control the silicone oil bath to 80°C, stabilize it for 10 minutes and then raise it to 100°C, and control the reflux The temperature is 100°C, after reflux for 36 hours, increase the temperature of the silicone oil bath, control the temperature at 110°C, reflux for 24 hours, stop heating; the product is found to be divided into two layers, the upper layer is the remaining n-butane chloride, and the lower layer is the target product Chloro-1 -Methyl-3-butylimidazole.
[0032] Quickly remove the remaining n-butane chlorid...
Embodiment 3
[0034] Embodiment 3: The synthesis of L-leucine-1-methyl-3-propylimidazole, compared with Example 1, the difference is that the raw material for the cation that provides the chiral ionic liquid is chloro-1-methyl Base-3-propylimidazole, the molar ratio of it and L-sodium leucine is 1:1, the steps of ion exchange reaction system, removal of NaCl and removal of acetone are the same.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com