Synthesis method of (R)-2-(2, 5-difluorophenyl) pyrrolidine
A synthesis method and difluorophenyl technology are applied in the synthesis field of -2-pyrrolidine, which can solve the problems of weak industrial production operability, poor chiral reduction selectivity, poor safety, and the like, and avoid the need for precious metal catalysts. and the use of metal organic compounds, the effect of good chiral purity and short steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
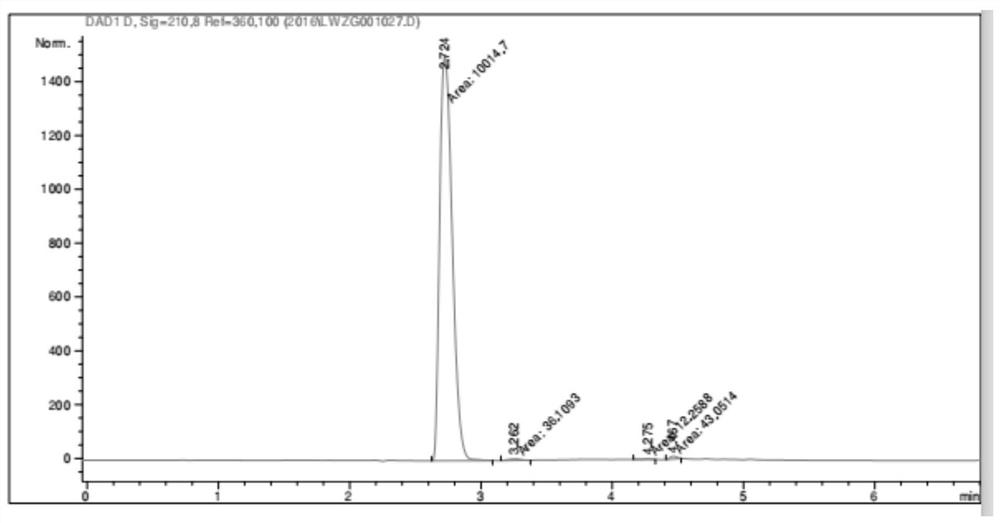
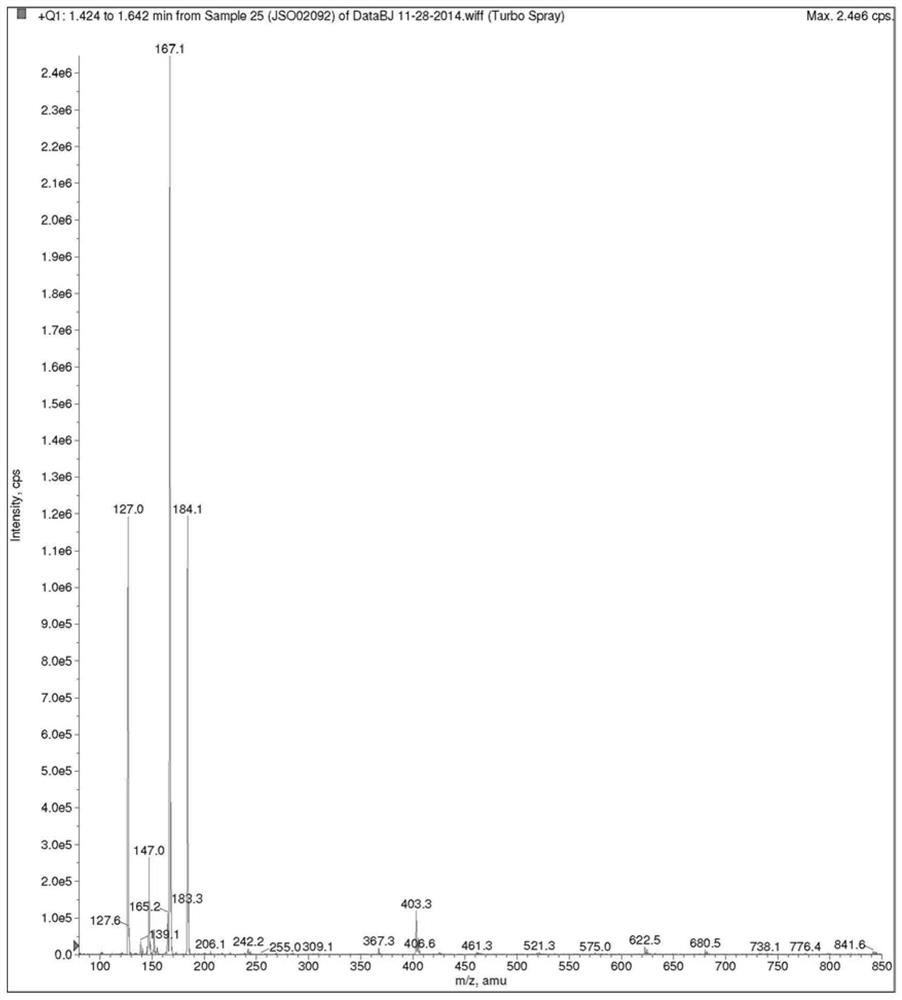
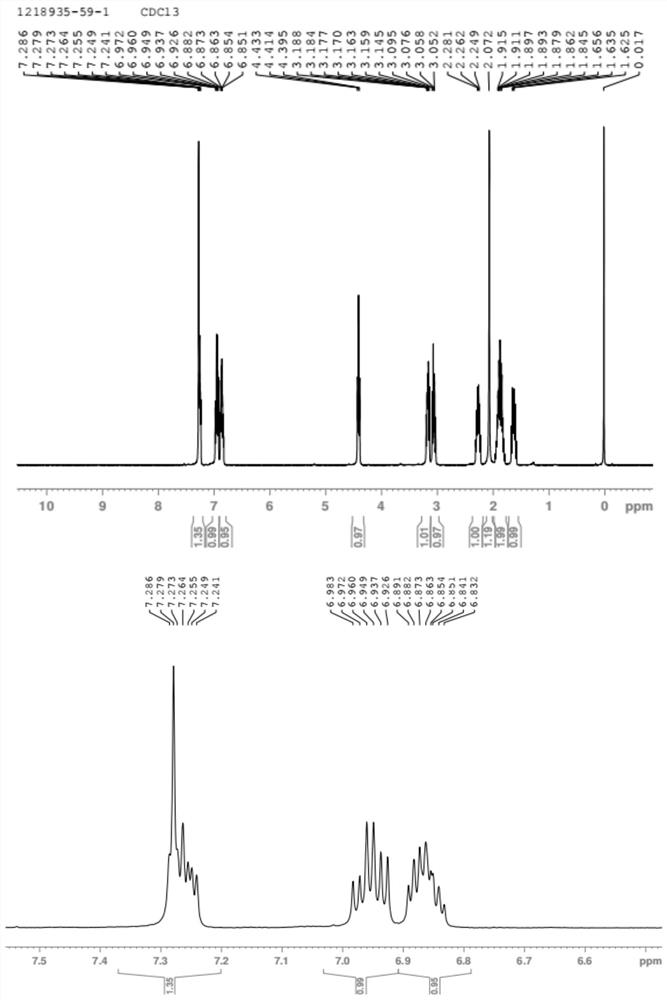
Image
Examples
Embodiment 1
[0077] Embodiment 1: the synthesis of formula 2 compound
[0078]
[0079] In a washed and dried three-necked flask, dissolve 60 grams of 2,5-difluorobenzaldehyde, 76.6 grams of R-tert-butylsulfinamide, 1.0 grams of PPTS, and 169 grams of anhydrous copper sulfate in 500 milliliters of dichloromethane , the oil bath was heated to 40 degrees Celsius, stirred, and refluxed for 3-5 hours. TLC / HPLC monitored the completion of the reaction. The product was filtered and concentrated under reduced pressure. After dissolving, wash with water and separate layers; wash with saturated brine, separate layers; dry over anhydrous sodium sulfate, and concentrate under reduced pressure to obtain 100.5 g of the compound Schiff base of formula 2 (light yellow oily substance), with a yield of 97%, which can be directly obtained without further purification. Carry out the next reaction.
Embodiment 2
[0080] Embodiment 2: the synthesis of formula 3 compound
[0081]
[0082] Dissolve 100.5 grams of Schiff base compound of formula 2 in 500 milliliters of tetrahydrofuran, under nitrogen protection, cool down to -30 degrees Celsius, slowly add 1.5 equivalents of 1 mol / L Grignard reagent dropwise, keep the temperature at about -30 degrees, after the addition is completed, keep the temperature constant After 2-3 hours of reaction, TLC / HPLC detected that the reaction was complete. Quench the reaction with saturated ammonium chloride aqueous solution, separate layers, wash with water, wash with saturated brine, dry over anhydrous sodium sulfate, and concentrate under reduced pressure to obtain 141.0 g of crude compound (light yellow oil) (DE>90%), yield>95 %.
Embodiment 3
[0083] Embodiment 3: the synthesis of formula 4 compound
[0084]
[0085] 141.0 grams of formula 3 compounds, stirred, slowly added the mixture of 3L trifluoroacetic acid and water (TFA:H 2 O volume ratio=95:5), stirred for 1-2 hours, and the temperature was controlled at 25 degrees Celsius. Slowly add 363 grams of triethylsilane dropwise, and control the temperature at 30-40 degrees Celsius. After the dropwise addition, control the temperature at 30 degrees Celsius and stir for 12-24 hours. HPLC detects that the reaction is complete. Concentration under reduced pressure afforded 106 g of crude compound (tan liquid). Without further purification, the next reaction can be carried out directly.
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com