Chiral poly (3,4-ethylenedioxythiophene) derivative monomer, polymer and preparation method thereof
A technology for ethylenedioxythiophene and derivatives is applied in the field of chiral poly(3,4-ethylenedioxythiophene) derivative monomers and polymers and their preparation, and achieves low cost, wide application prospects and high performance. excellent effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
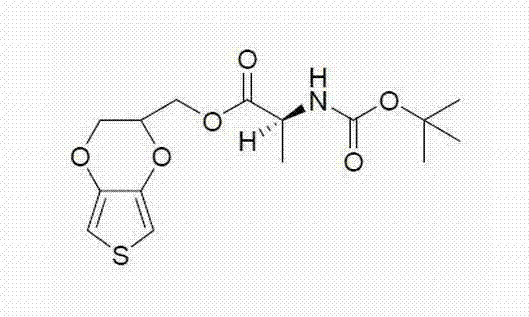
[0030] Embodiment 1: Synthesis of monomer compound EDOT-Boc-Ala
[0031] The synthesis scheme of monomeric EDOT-Boc-Ala is as follows:
[0032] .
[0033] Concrete synthetic steps are as follows:
[0034] 1, the synthesis of 3,4-dimethoxythiophene
[0035] in N 2 Under protection, 3,4-dibromothiophene (50.01 g, 206.67 mmol), copper oxide (16.50 g, 207.42 mmol) and potassium iodide (1.37 g, 8.25 mmol) were added to a 1000-mL three-necked flask, and the mass fraction 28% sodium methoxide solution (208.0 g, 1.08 mol) and 200 mL methanol. Reflux and stir for 96 h, TLC monitors the completion of the reaction, the reaction solution is cooled to room temperature and filtered, and the filter cake is washed with methanol. Pour the filtrate into 500 mL of water. Extract with dichloromethane, combine the organic phases and dry over anhydrous magnesium sulfate, remove the solvent by rotary evaporation under reduced pressure, and separate by silica gel column chromatography (petrol...
Embodiment 2
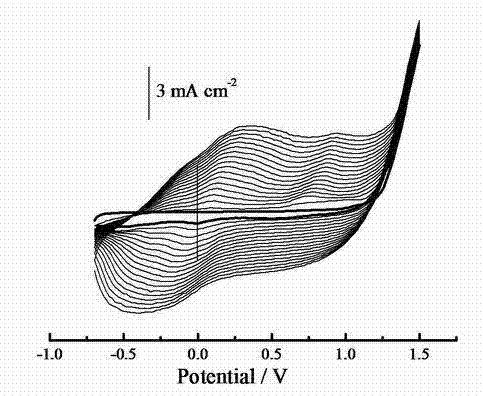
[0056] Example 2: Electrochemical Polymerization of PEDOT-Boc-Ala
[0057] Use refined dichloromethane as the electrolyte, the concentration is 0.02 mol L -1 EDOT-Boc-Ala is the reactive monomer, 0.1 mol L -1 Tetrabutylammonium boron tetrafluoride as the electrolyte. Stir evenly, and keep the solution in an argon atmosphere after protecting it with argon. at 20 o C. 25 o C. 30 o C and 35 o C. At four different temperatures, in a three-electrode system in which the platinum sheet is the working electrode and the counter electrode, and the platinum wire reference electrode, the constant potential polymerization is carried out under stirring. A working potential of 1.30 V was applied during polymerization. A blue-black solid can be obtained. The products prepared at four different temperatures are basically the same. The product is soaked in concentrated ammonia water for 3 days and dried. The specific rotation of the dedoped state is [α] 20.0 =-191.08°. Test the infra...
Embodiment 3
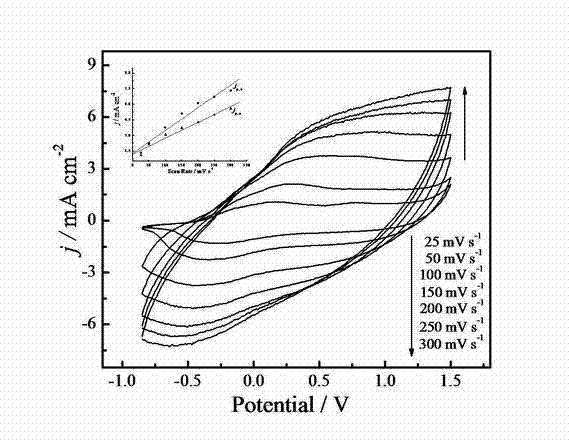
[0058] Example 3: Electrochemical Polymerization of PEDOT-Boc-Ala
[0059] The chiral PEDOT derivative was prepared according to the steps of Example 2, but the concentration of the monomer EDOT-Boc-Ala was 0.04 mol / L, and the same result was obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com