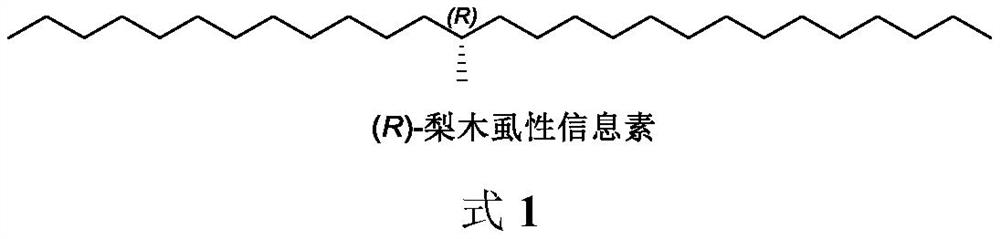
Method for synthesizing (R)-cacopsylla pyricola sex pheromone
A technology of psyllid and pheromone, which is applied in the field of new synthesis-sex pheromone of psyllid, can solve problems such as difficulty in amplification, cumbersome reaction steps, and lengthy routes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
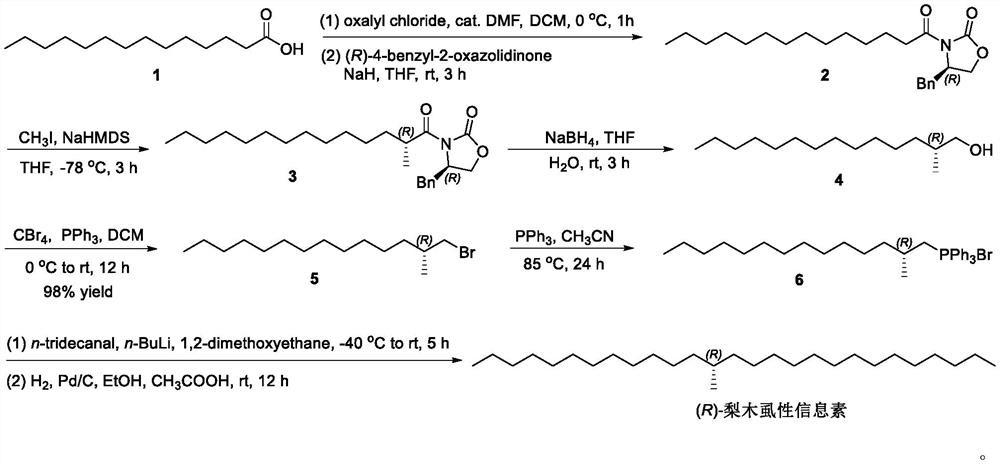
[0025] (R)-4-Benzyl-3-n-tetradecanoyl Synthesis of oxazolidinone (2)
[0026] At 0°C, DCM (20 mL), n-tetradecanoic acid 1 (2.28 g, 10 mmol) and 3-5 drops of DMF were sequentially added into a 100 mL three-neck flask, and mixed well. Then oxalyl chloride (1.90 g, 15 mmol) was slowly added dropwise. The reaction was stirred for 1 h at 0 °C. After TLC monitors that the reaction is complete, stop the reaction. Concentrate under reduced pressure to remove the reaction solvent to obtain crude n-tetradecanoyl chloride as light yellow solid.
[0027] At room temperature, sequentially add THF (20mL) and (R)-4-benzyl Oxazolidinone (1.45g, 8.25mmol), stirred well. Sodium hydride (0.50 g, mass fraction 60%, 12.3 mmol) was slowly added at 0° C., the mixture was warmed up to room temperature, and stirred for 2 h. The crude product of n-tetradecyl chloride was added at room temperature, and the stirring reaction was continued for 3h. After TLC monitors that the reaction is complete,...
Embodiment 2
[0029] (R)-4-Benzyl-3-((R)-2-methyltetradecanoyl) Synthesis of oxazolidinone (3)
[0030] Under argon protection, add in a 250mL three-necked bottle at room temperature Solution of oxazolidinone amide 3 (3.87 g, 10 mmol) in THF (80 mL). The solution was cooled to -78°C, NaHMDS (7.12 mL, 2.0M THF solution, 14.25 mmol) was added dropwise within 15 min, and the mixture was stirred for 1 h. At 78°C, iodomethane (7.34g, 3.22mL, 52mmol) was slowly added dropwise, and the stirring reaction was continued for 3h. After TLC monitors that the reaction is complete, stop the reaction. with saturated NH 4 Cl solution (75 mL) was used to quench the reaction, and the layers were separated. The aqueous phase was extracted with ethyl acetate (3 x 75 mL), and the organic phases were combined. The organic phase was washed with saturated NaCl solution (300 mL), anhydrous NaCl 2 SO 4 Dry and concentrate under reduced pressure to obtain crude product. The crude product was purified by sil...
Embodiment 3
[0032] Synthesis of (R)-2-methyl-1-tetradecyl alcohol (4)
[0033] Add in a 250mL three-necked bottle at room temperature Oxazolidinone amide 3 (8.90g, 22.16mmol) and THF (80mL), stirred and dissolved. Under ice-bath cooling, sodium borohydride (3.35 g, 88.65 mmol) aqueous solution (10 mL) was added dropwise within 30 min. The temperature of the reaction solution was raised to room temperature, and the stirring reaction was continued for 3 h. After TLC monitors that the reaction is complete, stop the reaction. Adjust the pH to 6 with 2.5M hydrochloric acid, and separate the liquids. The aqueous layer was extracted with ethyl acetate (3 x 30 mL), and the organic phases were combined. The organic phase was sequentially washed with saturated NaHCO 3 Aqueous solution (20mL) was washed with saturated NaCl solution (20mL), anhydrous NaCl 2 SO 4 Dry and concentrate under reduced pressure to obtain crude product. The crude product was purified by silica gel column chromatogra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com