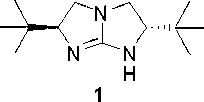
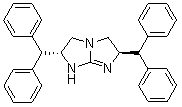
Chiral five-membered bicyclic guanidine compound, preparation method and application thereof
A technology of compound and bicyclic guanidine, which is applied in the field of chiral five-membered bicyclic guanidine compound and its preparation, can solve the problems of catalyst application limitation, low reaction yield, and inability to carry out the reaction, and achieve good asymmetric reaction catalytic performance, good The effect of applying the foreground
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1 Preparation of chiral five-membered bicyclic guanidine compound of formula (I)
[0037] (1) In a flask, add benzene (5.0 ml) and trifluoromethanesulfonic acid (20.0 ml), add Amino alcohol 3 (1.0 g), stirred for 3 to 6 hours. The reaction solution was poured into about 100 g of ice, stirred thoroughly, neutralized with sodium hydroxide solution, and extracted with chloroform. The organic phase was washed successively with water and saturated brine, and finally dried over magnesium sulfate, filtered, and the solvent was removed under reduced pressure to obtain Amino alcohol 4 The crude product (1.29 g, 96% yield). The crude product can be directly used in the next reaction.
[0038] (2) Add in the flask Amino alcohol 4 (1 g), triethylamine (2.1 ml) and dry acetonitrile (11 ml), stirred and cooled to 0~5°C in an ice bath. Add p-toluenesulfonyl chloride (0.8 g), stir in an ice bath for half an hour, naturally warm to room temperature, and stir for another...
Embodiment 2
[0044] Embodiment 2 catalytic experiment
[0045] Below, we provide examples of the application of the compound of formula (I) to catalyze the Michael addition reaction of 2-cyclopentenone and dimethyl malonate.
[0046] 2-Cyclopentenone (0.025 mmol) and dimethyl malonate (0.03 mmol, 1.2 eq) were dissolved in toluene (0.1 ml), and stirred at room temperature. The compound catalyst (1.1 mg, 0.0025 mmol, 0.01 equivalent) described in formula (I) was dissolved in 0.01 ml of toluene, added to the reactant, stirred, and the progress of the reaction was monitored by thin layer chromatography. After stirring at room temperature for 6 days, the reaction was stopped and the product was separated by column 12 . (2.94 mg, 55% yield).
[0047]
[0048] to detect Product (S)-(-)-12 The chiral purity of the product can be transformed into Acetal (S)-13 Afterwards, the chiral purity was analyzed by chiral column chromatography. The conditions are: CHIRALCEL OD-H (4.6 mm i.d. x 2...
Embodiment 3
[0051] Embodiment 3 catalytic performance comparative experiment
[0052] The Michael addition reaction between nitrostyrene and dimethyl malonate is widely used in the synthesis of chiral molecules.
[0053] Unlike the cyclic substrate of 2-cyclopentenone in Example 2, a nitrostyrene is actually a linear substrate. However, due to Compound 1 The catalytic performance or catalytic conditions are limited, and have not yet been used Compound 1 Report on the successful catalyzed Michael addition of nitrostyrene to dimethyl malonate. We have carried out the following experiments with the compound of formula (I) as catalyst:
[0054] Nitrostyrene (0.025 mmol) and dimethyl malonate (0.03 mmol, 1.2 equiv) were dissolved in toluene (0.1 ml), and stirred at room temperature. The compound catalyst (1.1 mg, 0.0025 mmol, 0.01 equivalent) described in formula (I) was dissolved in 0.01 ml of toluene, added to the reactant, stirred, and the progress of the reaction was monitored by t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com