Ionic compound containing chiral amine-thiourea (urea) and its preparation method and application
A technology of ionic compounds and chiral amines, applied in the directions of organic chemistry methods, chemical instruments and methods, organic compounds/hydrides/coordination complex catalysts, etc. , The effect of wide application and low dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1: Preparation of Chiral Ionic Compound 1
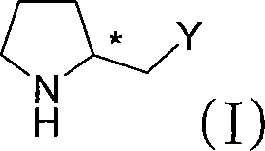
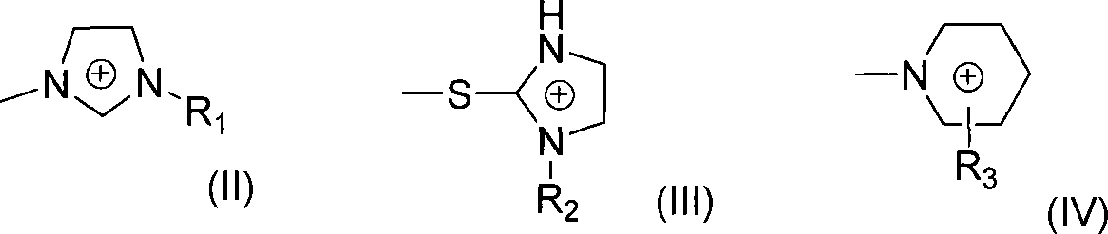
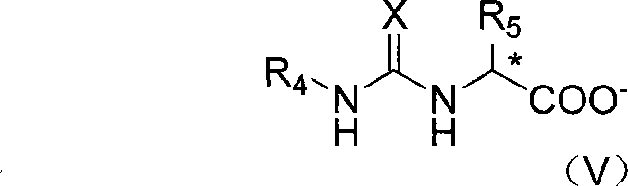
[0035] Add 3-methyl-2-((S)-pyrrolidinyl-2-methylthio)-3H-imidazolium bromide (1mmol), (3-phenyl-thioureido) sodium acetate into a 100mL Erlenmeyer flask (1mmol), THF (60mL) and water (30mL), stirred at room temperature for 24h, after the reaction was complete, desolvated under reduced pressure, then the solid was dissolved in 20mL of chloroform, filtered to remove inorganic salts, and the filtrate was distilled to remove the solvent to obtain The target compound (94% yield). Its specific rotation [α] D 20 =+45°.
Embodiment 2
[0036] Embodiment 2: Preparation of chiral ionic compound 2
[0037] Add 3-methyl-2-((S)-pyrrolidinyl-2-methylthio)-3H-imidazolium bromide (1mmol), (S)-3-phenyl-2- Sodium (3-phenyl-thioureido)propionate (1mmol), ethanol (10mL), stirred at room temperature for 24h, after the reaction was complete, the solid was dissolved in 20mL of chloroform, filtered to remove inorganic salts, and the filtrate was distilled off The target compound was obtained after solvent removal (yield 93%). Its specific rotation [α] D 20 =+28.4°.
Embodiment 3
[0038] Embodiment 3: Preparation of chiral ionic compound 3
[0039]Add 3-methyl-2-((S)-pyrrolidinyl-2-methylthio)-3H-imidazolium bromide (1mmol), (R)-3-phenyl-2-( 3-Phenyl-thioureido)sodium propionate (1mmol), methanol (60mL), stirred at room temperature for 24h, after the reaction was complete, desolvated under reduced pressure, then dissolved the solid in 20mL of chloroform, filtered to remove inorganic salts, and then The filtrate was distilled to remove the solvent to obtain the target compound (95%). Its specific rotation [α] D 20 =+28.4°.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific rotation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com