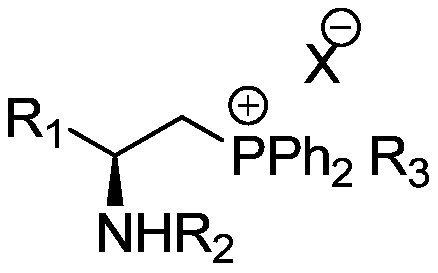
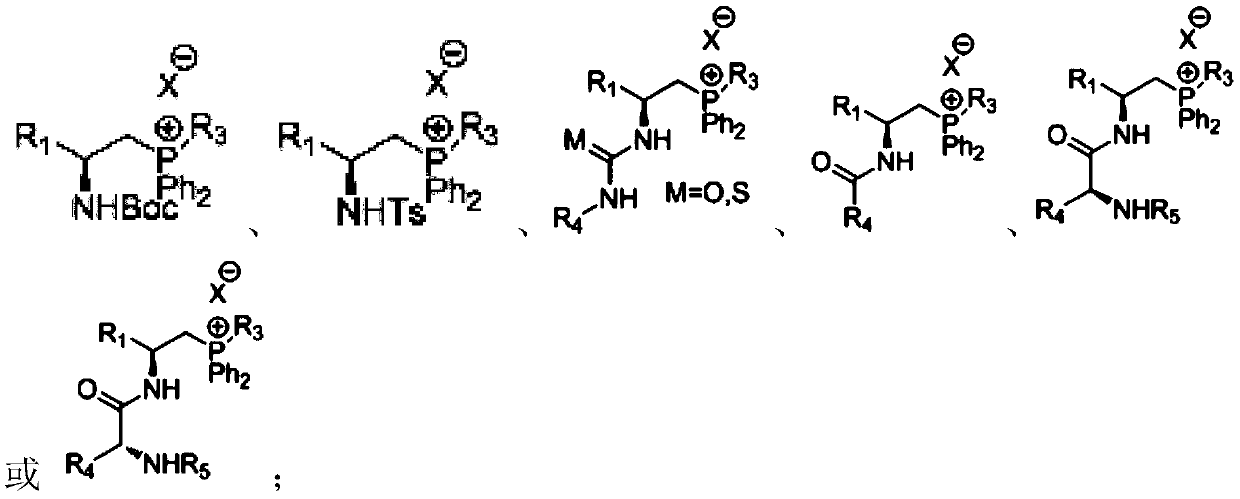
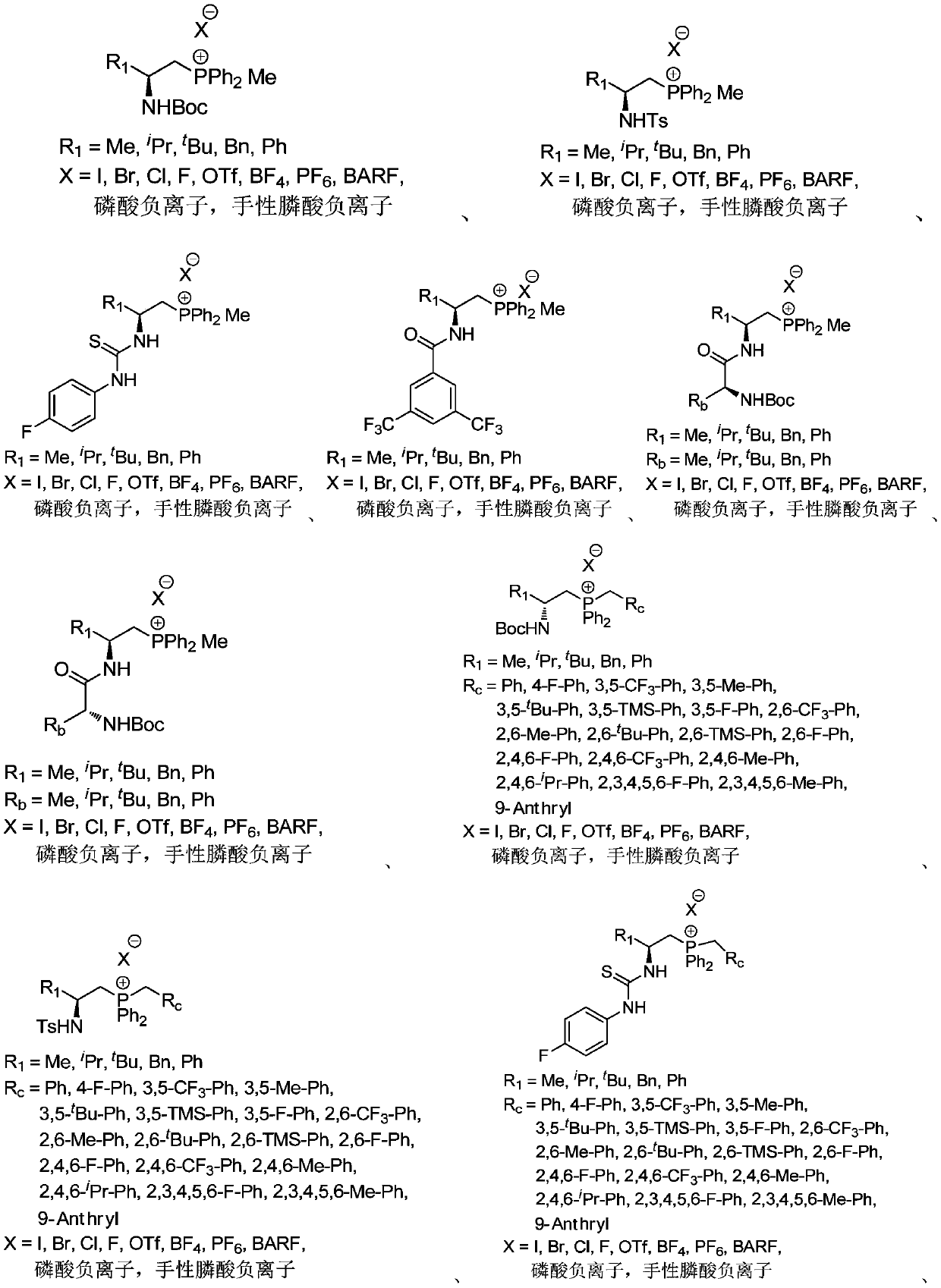
Chiral quaternary phosphonium salt phase transfer catalyst, preparation method and application thereof
A technology of phase transfer catalysts and quaternary phosphonium salts, which is applied in organic chemical methods, chemical instruments and methods, physical/chemical process catalysts, etc., can solve the problems of few catalytic examples, achieve stable air and water performance, diverse structures, and easy The effect of the operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] (S)-Methyl(3-methyl-2-(4-methylbenzenesulfonamide)butyl)diphenyl iodide , the structural formula is:
[0050]
[0051] Concrete preparation process is as follows:
[0052] (1) Reflux reaction of valine with hydrochloric acid / methanol solution for 5 hours, spin the solvent, then add TsCl and triethylamine, react at room temperature for 3 hours, and obtain compound 2 through extraction and concentration; wherein, valine, TsCl and triethylamine The molar ratio of ethylamine is 1:1.2:2;
[0053] (2) Dissolve compound 2 in an organic solvent, then add LiAlH 4 React at room temperature for 3 h, filter, and react the filtered product with EsCl and triethylamine for 3 h to obtain compound 3; wherein, compound 2, LiAlH 4 , the mol ratio of EsCl and triethylamine is 1:2:1.2:2;
[0054] (3) Compound 3 was added to NaOH aqueous solution, stirred at room temperature for 3 h, extracted and concentrated to obtain Compound 4;
[0055] (4) Compound 4 and KPPh 2 Mix according to t...
Embodiment 2
[0061] ((S)-2-(S)-2-((tert-butoxycarbonyl)amino)-3-methylbutylamine)-3-methylbutyl(methyl)(methyl) Diphenylammonium iodide , the structural formula is:
[0062]
[0063] The specific preparation process is as follows: according to the preparation method of Example 1, its starting material is also valine, and the replacement group is replaced according to the above structure in the reaction process, and finally tert-butyl-1-(((s) -1-(diphenylphosphine)-3-methylbutan-2-yl)amino)-3-methyl-1-oxobutan-2-yl)carbamate,
[0064] Add tert-butyl-1-(((s)-1-(diphenylphosphine)-3-methylbutan-2-yl)amino)-3-methyl-1-oxobutane to the reaction flask -2-yl) carbamate 470mg (1.0mmol), iodomethane 170mg (1.2mmol) and 20ml tetrahydrofuran, reacted at room temperature for 2h, and concentrated to obtain 575.3mg of the product with a yield of 94%.
[0065] NMR and MS data: 1 H NMR (400MHz, CD 3 OD)δ8.00-7.79(m,6H),7.77-7.67(m,4H),4.21-4.05(m,1H),3.63(d,J=6.2Hz,1H),3.50-3.35(m,1H ), 3.24(td...
Embodiment 3
[0067] ((S)-2-((R)-2-((tert-butoxycarbonyl)amino)-3-methylbutylamine)-3-methylbutyl(methyl)(methyl) Diphenylammonium iodide , the structural formula is:
[0068]
[0069] Concrete preparation process is as follows:
[0070] According to the preparation method of Example 1, the starting material is also valine, and the replacement group is replaced according to the above structure in the reaction process, and finally tert-butyl-1-(((s)-1-(di Phenylphosphine)-3-methylbutan-2-yl)amino)-3-methyl-1-oxobutan-2-yl)carbamate, add tert-butyl-1- (((s)-1-(diphenylphosphine)-3-methylbutan-2-yl)amino)-3-methyl-1-oxobutan-2-yl)carbamate 470mg( 1.0 mmol), 170 mg (1.2 mmol) of methyl iodide and 20 ml of tetrahydrofuran, reacted at room temperature for 5 h, and concentrated to obtain 599.3 mg of the product, with a yield of 98%.
[0071] NMR and MS data: 1 H NMR (400MHz, CD 3 OD)δ7.94-7.77(m,6H),7.75-7.65(m,4H),4.20-4.04(m,1H),3.74(d,J=5.8Hz,1H),3.37-3.19(m,2H ), 2.72(d, J=14.0Hz, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com