Method for synthesizing amino acids with large steric hindrance and oligopeptides through palladium catalytic carboxylic acid guided arylation
A technology for catalyzing carboxylic acids and amino acids, applied in peptide preparation methods, chemical instruments and methods, peptides, etc., can solve problems such as limited modification range, and achieve high activity, high efficiency, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
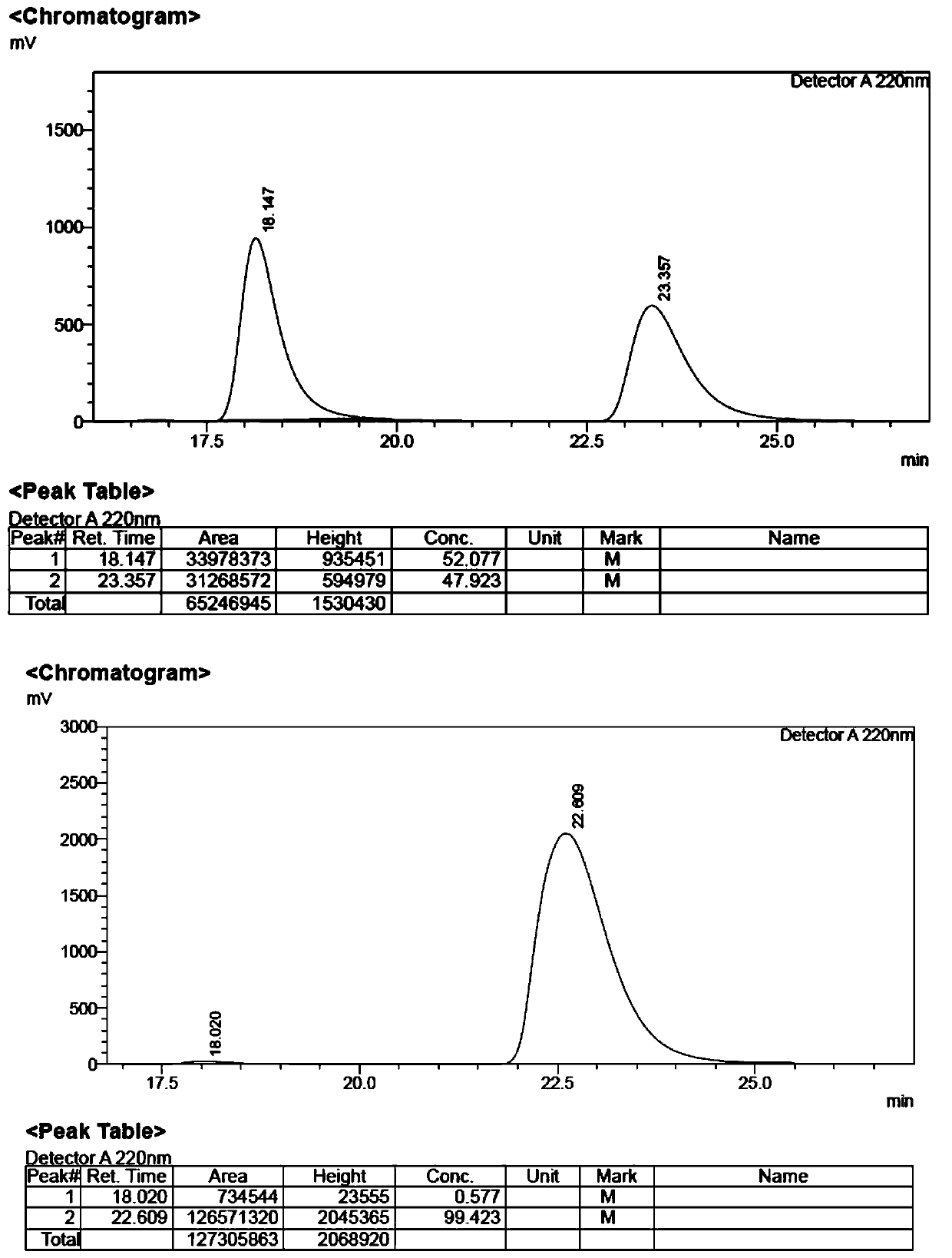
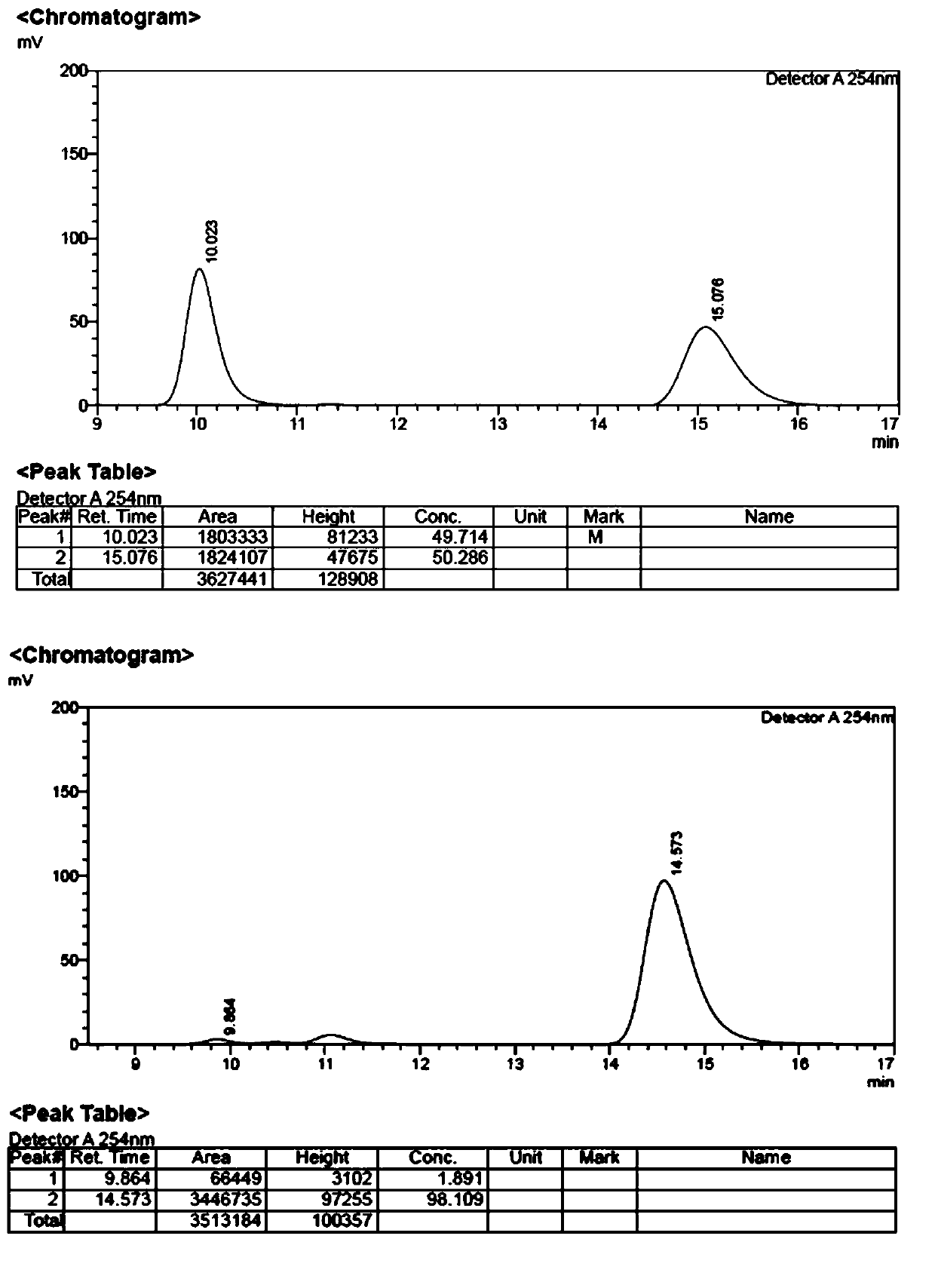
[0033] In the reactor, add 0.3 mmol of phthaloyl-protected L-type tertiary amino acid, 0.9 mmol of 4-methoxyiodobenzene, 0.03 mmol of palladium acetate catalyst, 0.3 mmol of silver phosphate, 0.3 mmol of acetyl Tert-leucine, 0.3 mmol potassium carbonate and 3 milliliters of hexafluoroisopropanol were reacted in an air atmosphere at 70° C. for 24 hours, and then the reaction was finished for post-treatment, and product 1 and product 1′ were obtained by thin-layer silica gel plate chromatography. The rates were 58% and 10%, respectively, and the ee values were 99% and 96%. The HPLC spectrum of compound 1, 1' and its racemate is shown in figure 1 , figure 2 .
[0034] The structure of product 1 is as follows (wherein, Phth is phthaloyl):
[0035]
[0036] The structural characterization data are as follows:
[0037] 1 H NMR (400MHz, CDCl 3 )δ7.92–7.82(m,2H),7.79–7.70(m,2H), 7.09(d,J=8.6Hz,2H),6.82(d,J=8.6Hz,2H),4.86(s,1H ),3.79(s,3H), 3.01(d,J=13.3Hz,1H),2.83(d,J=13....
Embodiment 2~15
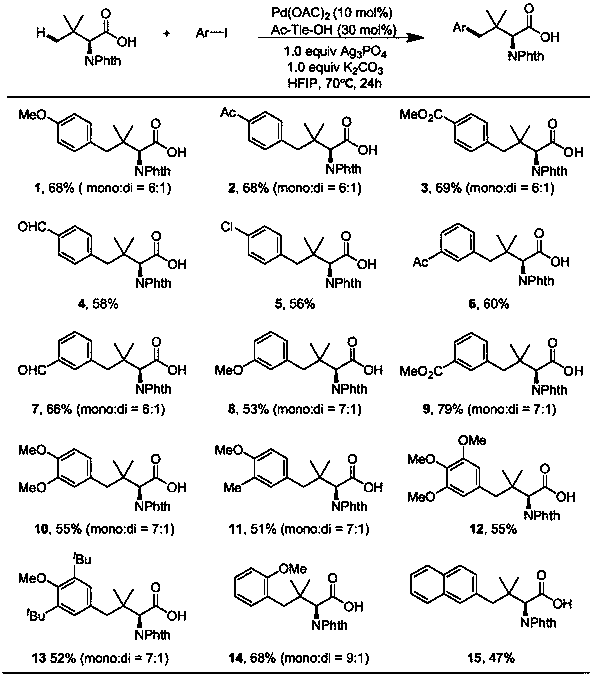
[0044] The operation steps are the same as in Example 1, the difference is that by changing the substituent on the raw material iodobenzene, different arylated products can be obtained (see Table 1).
[0045] Table 1 Embodiment 2~15 synthetic big sterically hindered amino acid experimental result
[0046]
Embodiment 16
[0048] Add 0.3 mmol L-type 3-bromophthaloyl tertiary amino acid, 0.9 mmol 4-methoxyiodobenzene, 0.03 mmol palladium acetate catalyst, 0.3 mmol silver phosphate, 0.3 mmol acetyl tertiary leucine , 0.3 millimoles of potassium carbonate and 3 milliliters of hexafluoroisopropanol, reacted in an air atmosphere at 70 ° C for 24 hours and then finished the reaction for post-treatment, and obtained product 16 and product 16 'yields were 61% by thin-layer silica gel plate chromatography. %, 15%.
[0049] The structure of product 16 is as follows:
[0050]
[0051] Product characterization data are as follows:
[0052] 1H NMR (400MHz, CDCl3) δ7.99(s, 1H), 7.87(d, J=7.7, 1H), 7.72(d, J=7.9Hz, 1H), 7.08(d, J=8.2Hz, 2H) ,6.82(d,J=8.1Hz,2H),4.83(s,1H), 3.79(s,3H),3.00(d,J=13.3Hz,1H),2.81(d,J=13.3Hz,1H) ,1.16(s,3H), 0.99(s,3H).13C NMR(101MHz,CDCl3)δ173.09,167.22,166.68, 158.17,137.32,134.29,133.23,131.82,130.11,129.69,129.31,712.504, 6 113.36,55.20,44.56,39.34,28.01,24.78,24.64.HRMS(E...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com