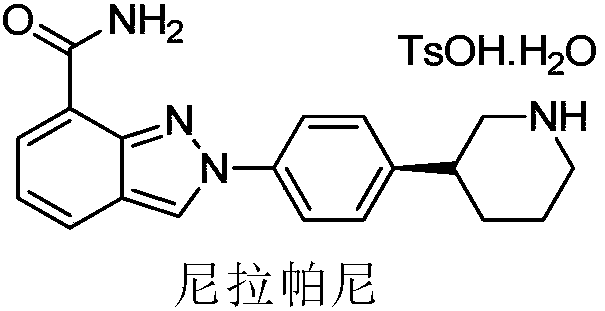
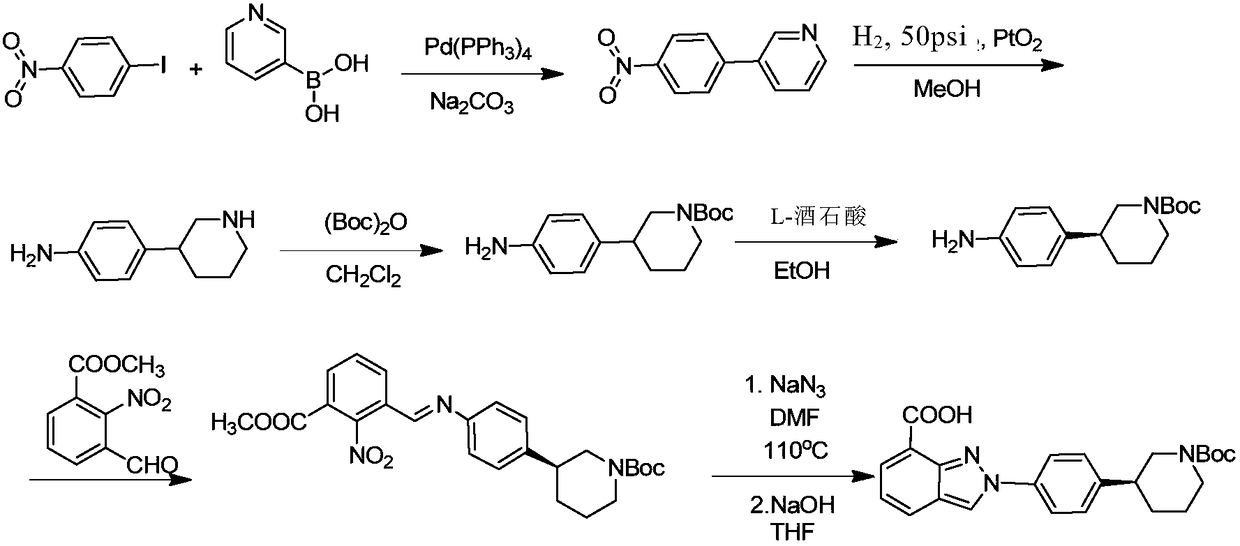
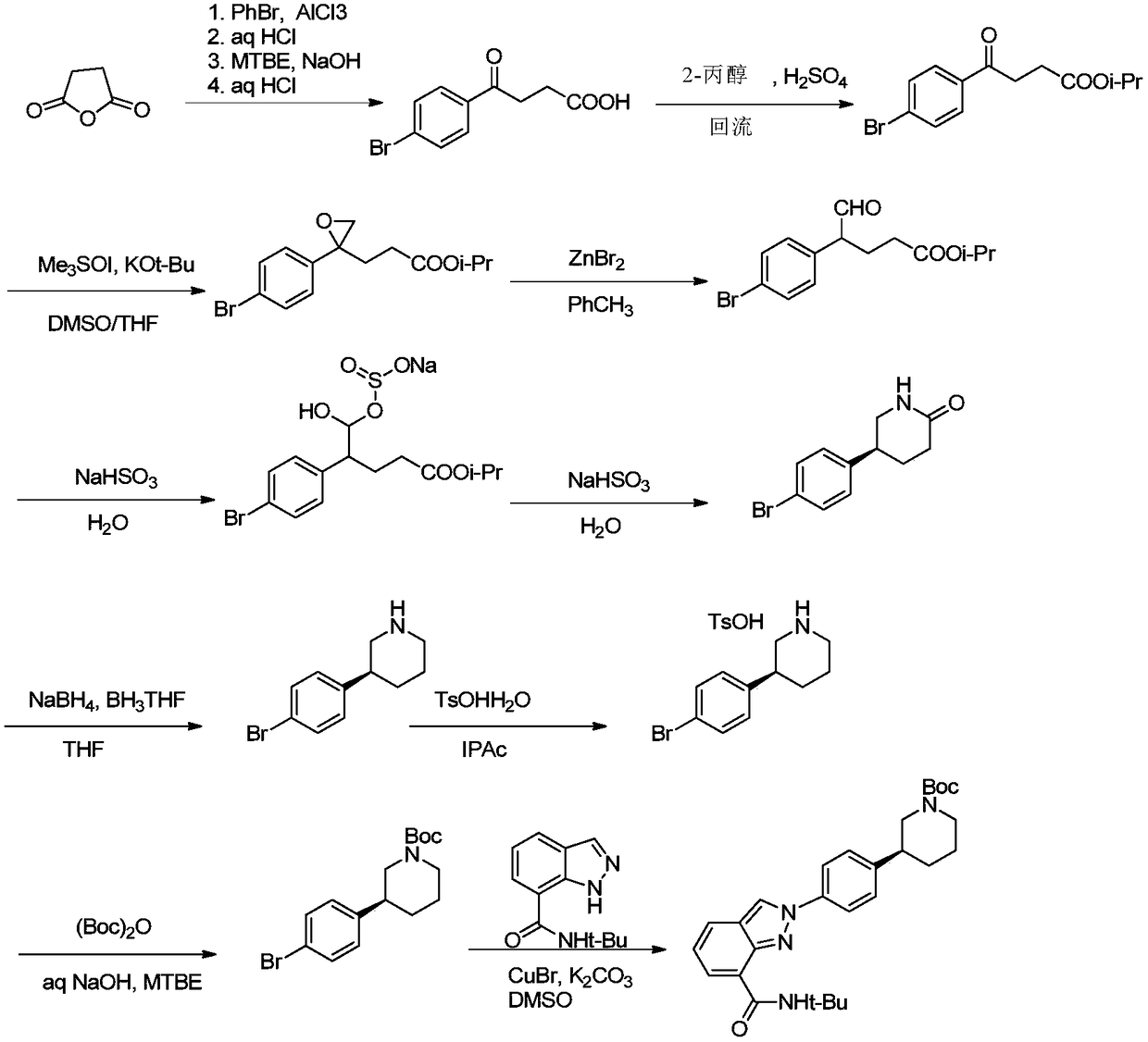
Method of synthesizing (S)-3-(4-bromophenyl)-piperidine or salt thereof with chiral induction
A synthesis method and bromophenyl technology are applied in the field of chiral induced synthesis of -3--piperidine or its salts, and can solve the problems of large three wastes, long synthesis steps, large amount of raw materials and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0088] The invention provides a method for preparing (S)-3-(4-bromophenyl)-piperidine or a salt thereof, the method comprising the following steps (1)-(6).
[0089] Step (1): Condensation reaction between compound 1 and (S)-(-)-tert-butylsulfinamide in the presence of a catalyst under solvent-free conditions. After the reaction, concentrate the reaction mixture and collect the concentrate , so as to obtain compound 2 or a product containing compound 2;
[0090]
[0091] In step (1), the function of the concentration is generally to remove the unreacted by-product (methanol) and recover the raw material compound 1 (trimethyl-4-bromophenylacetate). The concentration is preferably concentration under reduced pressure. The pressure of the reduced-pressure concentration is preferably 0.5 mmHg. The concentration temperature is preferably 60 to 100°C, more preferably 70 to 75°C.
[0092] In step (1), the condensation reaction is a solvent-free reaction.
[0093] In step (1), t...
Embodiment 1
[0162]
[0163]In a dry three-necked round bottom flask of 100ml, the reactant ortho-4-bromobenzoic acid trimethyl ester 1 (41.3g, 150mmol, 1.5eq) was dropped, followed by the raw material (S)-(-)-tert-butylsulfinic acid Amide (11.2g, 100mmol, 1.0eq) and catalyst p-toluenesulfonic acid (0.34g, 2mmol, 0.02eq), magnetically stirred. Heat the internal temperature of the system to 90-95 degrees and reflux for 3 hours. After the reaction is complete, distill the fraction below 75 degrees under reduced pressure (0.5mm Hg) for subsequent batches. Cool the remaining system to room temperature to obtain 49.3g of Crude Compound 2. The yield was 99.0%, and it was directly carried out to the next reaction without further purification. 1 H NMR (400MHz, CDCl 3 ): δ1.21(9H,s),3.74(3H,s),3.98(2H,dd),7.21-7.23(2H,m),7.42-7.44(2H,m).
Embodiment 2
[0165]
[0166] The crude compound 2 (33.2 g, 100 mmol, 1.0 eq) obtained in Example 1 and 250 ml of anhydrous tetrahydrofuran as a reaction solvent were put into a 1000 ml dry three-neck round bottom flask. After 3 nitrogen replacements, cool to -70~-65 degrees, add 1 mol / L LiHMDS solution (200ml, 200mmol, 2eq) dropwise at this temperature, drop it within 1 hour, and continue to stir and react at this temperature for 1 hour. 1-Chloro-3-iodopropane (26.6g, 130mmol, 1.3eq) was added dropwise at this temperature, and after the dropwise addition was completed for 1 hour, it was stirred at -70~-65°C for 2 hours. Then the internal temperature of the system was heated to -20°C, and 250 ml of saturated ammonium chloride aqueous solution was added dropwise, and the dropping temperature was controlled at -20 to -15°C. After the dropwise addition, stir for 15 minutes, add 100 ml of ethyl acetate for extraction three times, combine the organic phases, and wash the organic phases once w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com