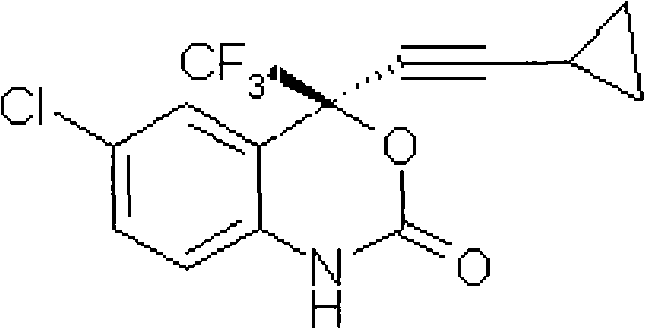
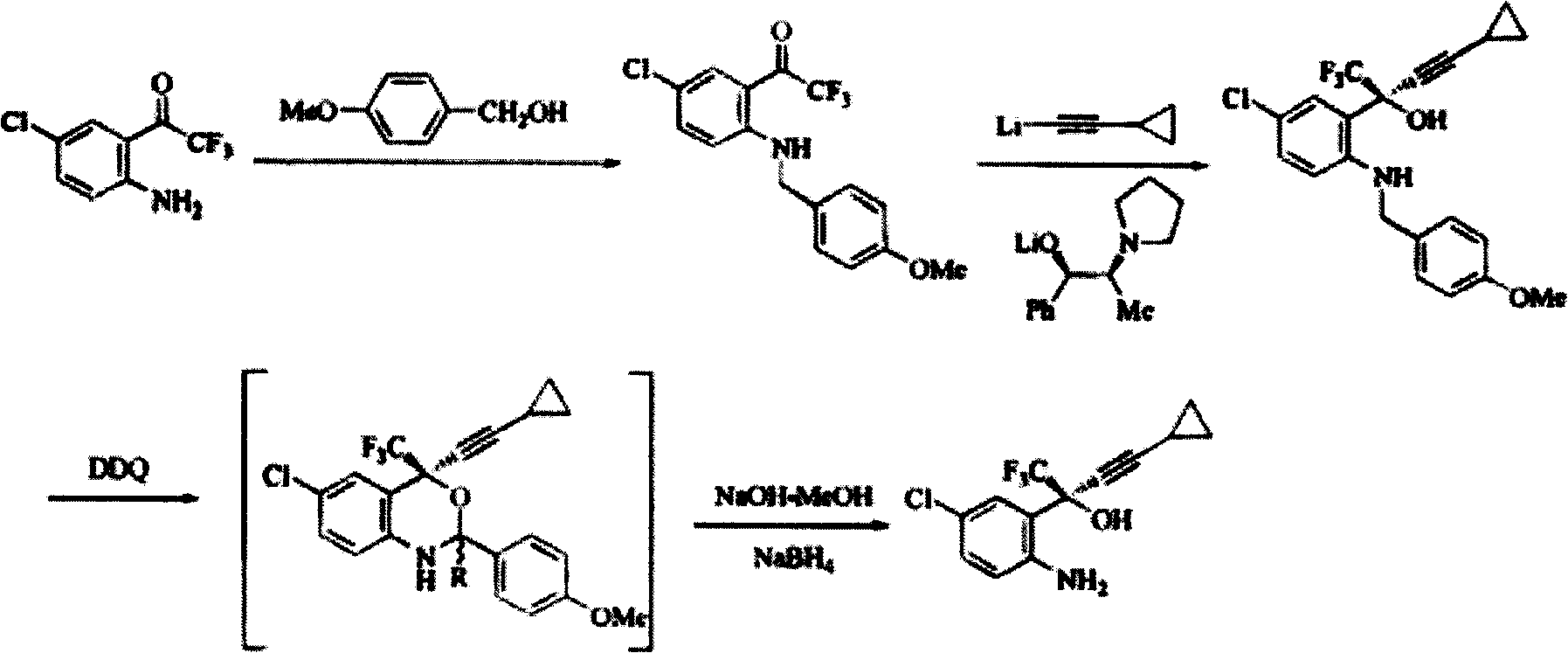
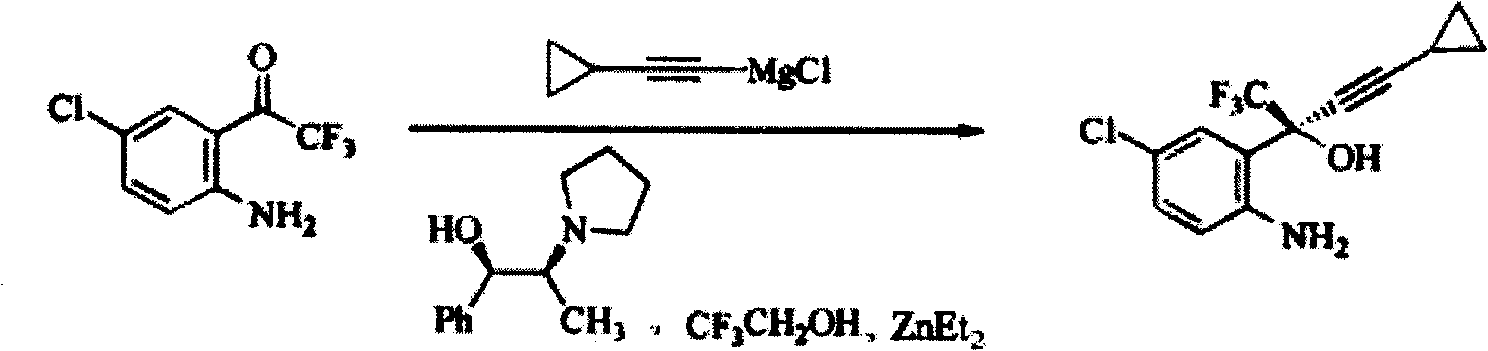Method for preparing chiral cylopropyl acetenyl tertiary alcohol compound
A technology for cyclopropylacetylene and compounds, applied in the field of preparation of -2-amino-5-chloro-α-cyclopropylacetylene-α-trifluoromethylbenzyl alcohol, which can solve burns, high cost, high price, etc. problem, to achieve the effect of cost reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Preparation of cyclopropynemagnesium chloride:
[0039] After the reaction bottle was fully replaced with argon, 4.64ml of cyclopropyne was added, cooled to 0°C, and 28.6ml of n-butylmagnesium chloride was slowly added dropwise, and the internal temperature was controlled at 15°C. After the dropwise addition, continue to stir at 0°C for 2 hours to obtain cyclopropyne magnesium chloride.
Embodiment 2
[0041] After the reaction flask was fully replaced with argon, 90ml of dry methyl tert-butyl ether and NaH (6.0g) were added, cooled to 10°C, trifluoroethanol (3.1ml) was added dropwise, and the internal temperature was controlled at 25°C. Cool to 20°C after dropwise addition, add (1R,2S)-N-pyrrolidinylnorephedrine (13.5g), stir at 25°C for 1 hour, add zinc chloride (9.0g), stir at 25°C 1 hour. 28.6 ml of tetrahydrofuran solution of cyclopropyne magnesium chloride (1.8 M) obtained in Example 1 was added dropwise, and the inner temperature was kept at 25°C. The addition was completed in about 5 minutes, and was added after washing with 1 ml of tetrahydrofuran. After the addition was complete, stir at 25°C for 1 hour. Then cool to -20°C and add 5-chloro-2-aminotrifluorobenzophenone (10.0g), react at this temperature for 1 hour, raise the temperature to -10°C for 3 hours, then slowly raise the temperature to 0°C for 3 hours, Finally, the temperature was naturally raised to roo...
Embodiment 3
[0043] After the reaction bottle was fully replaced with argon, 100ml of dry toluene and NaH (6.0g) were added, cooled to 10°C, trichloroethanol (5.3ml) was added dropwise, and the internal temperature was controlled at 25°C. Cool to 20°C after dropwise addition, add (1R,2S)-N-pyrrolidinylnorephedrine (13.5g), stir at 25°C for 1 hour, add zinc chloride (10.0g), stir at 25°C 1 hour. 28.6 ml of tetrahydrofuran solution of cyclopropyne magnesium chloride (1.8 M) obtained in Example 1 was added dropwise, and the inner temperature was kept at 25°C. The addition was completed in about 5 minutes, and was added after washing with 1 ml of tetrahydrofuran. After the addition was complete, stir at 25°C for 1 hour. Then cool to -20°C and add 5-chloro-2-aminotrifluorobenzophenone (10.0g), react at this temperature for 2 hours, heat up to -5°C for 3 hours, then slowly heat up to 5°C for 3 hours, Finally, the temperature was naturally raised to room temperature for 4 hours. After the rea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com