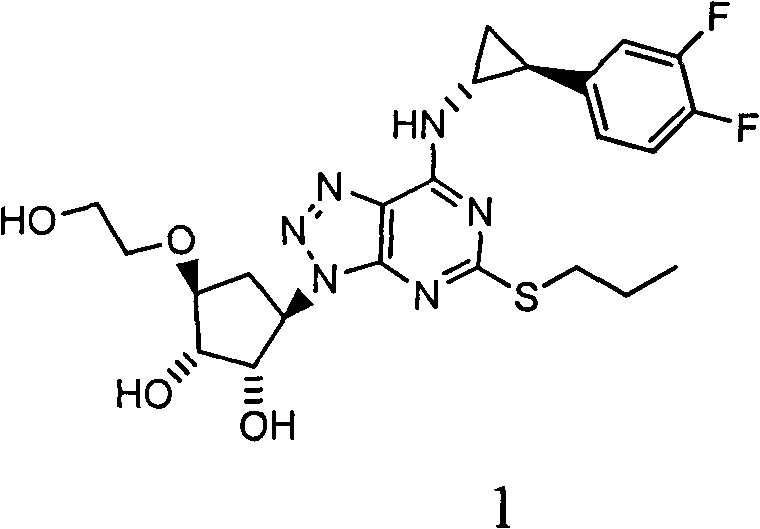
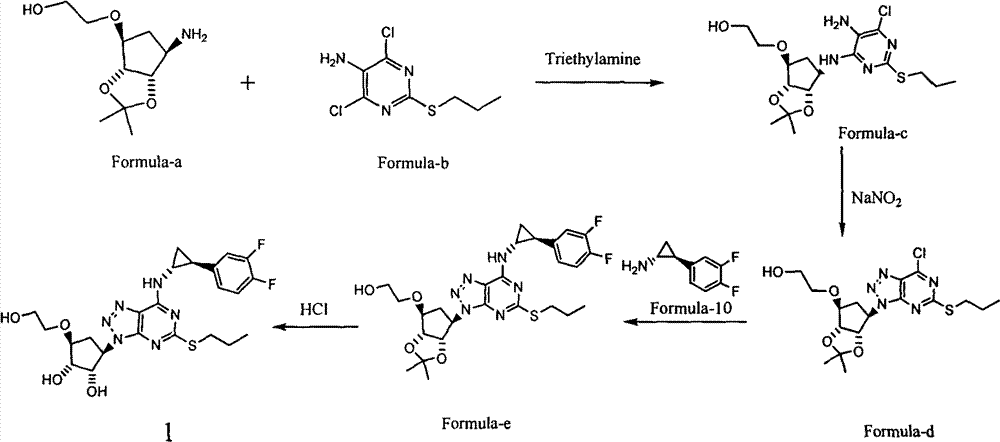
Novel preparation method of antithrombosis medicine
A compound, the technology of ticagrelor, which is applied in the new preparation field of ticagrelor, can solve the problems of small chlorine group activity, dark color, and many side reactions, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
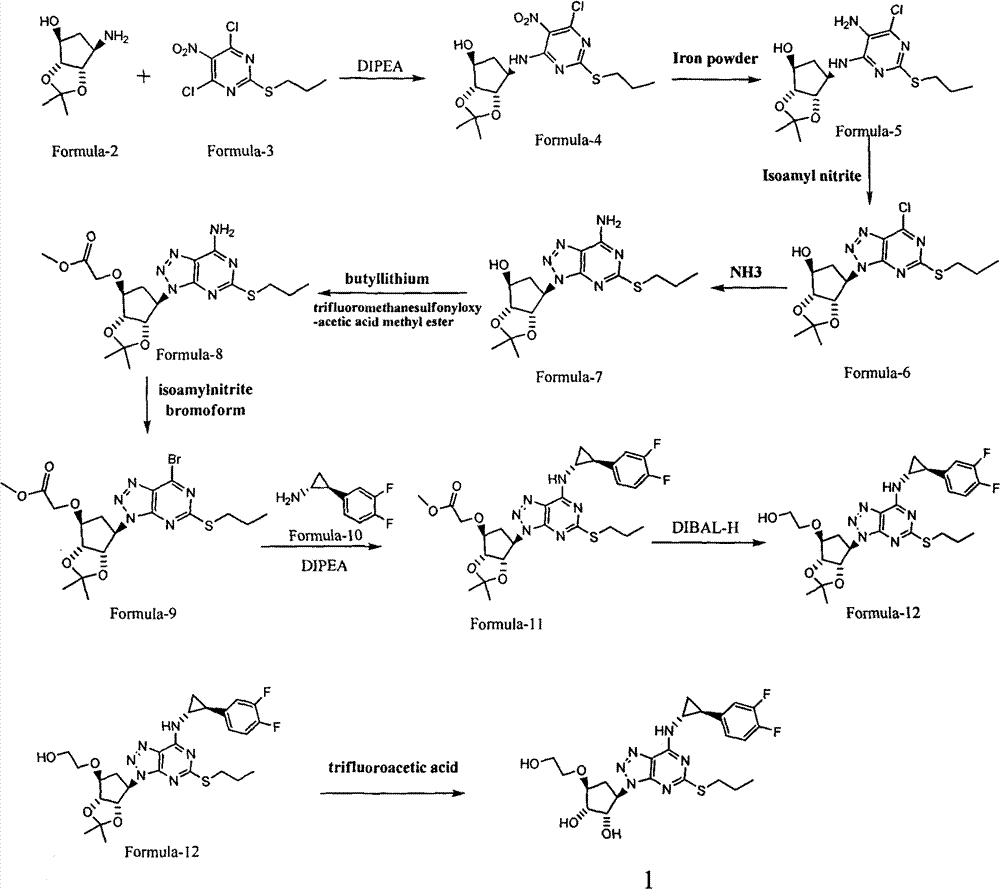
Examples
Embodiment 1
[0074] Synthesis of formula Formula-E compound (R is ethyl in the structural formula):
[0075] Add 60g (0.23mol, 1.0eq) of the Formula-D compound (R in the structural formula is ethyl, see US2003 / 0148888 for the preparation method), 140mL (0.74mol, 3.0eq) of DIPEA into 900mL of THF, and stir at room temperature for 0.5 hours. use. Add 99.2g (0.37mol, 1.6eq) of Formula-3 compound into 180mLTHF, cool to 0-5°C, add the above-mentioned ready-to-use solution dropwise under nitrogen protection, and control the temperature at 0-5°C for about one hour. After the dropwise addition, keep the reaction system at 0-5° C. and stir for two hours. TLC detects that there is no remaining raw material. Add 250mL of ethyl acetate to dilute the solution, wash the organic phase with water (300mL), wash once with saturated brine (300mL), dry over anhydrous sodium sulfate, concentrate to obtain 150g of light yellow oil, add 500ml of dichloromethane to the oil and stir to dissolve Then add 300g of ...
Embodiment 2
[0078] Synthesis of formula Formula-F compound (R is ethyl in the structural formula):
[0079] 65g (132mmol) of the compound of formula F was dissolved in 70mL of methanol, and 6.5g of 10% palladium on carbon was added under the protection of nitrogen to replace the hydrogen. The reaction was carried out at room temperature and normal pressure for 20 hours, and there was no remaining raw material as detected by TLC. Palladium carbon was filtered, the filter cake was washed with 50 mL of methanol, and the filtrate was concentrated to obtain 57 g of oil, which was directly used in the next reaction without purification, with a yield of 93.4%.
[0080] NMR δH(CDCl 3 )6.32(1H, d, J=7.6Hz), 4.64(1H, t, J=7.2Hz), 4.57~4.54(2H, m), 4.33~4.20(3H, m), 4.12~4.07(1H, m ), 3.98(1H, d, J=4.0Hz), 3.76(2H, br s), 3.16~3.09(1H, m), 3.03~2.96(1H, m), 2.32~2.25(1H, m), 1.88 (1H, d, J=14.8Hz), 1.77~1.71(2H, m), 1.41(3H, s), 1.29(3H, t, J=7.2Hz), 1.24(3H, s), 1.01(3H, t, J=7.2Hz).
Embodiment 3
[0082] Synthesis of formula Formula-G compound (R is ethyl in the structural formula):
[0083] 52 g (113 mmol, 1.0 eq) of the compound of formula G were dissolved in 150 mL of acetonitrile. Add 30.4mL (226mmol, 2.0eq) of isoamyl nitrite, heat to 70°C and react for 1 hour, TLC detects that no raw material remains. After cooling the reaction to room temperature, it was concentrated to obtain 51.1 g of brown oil with a yield of 96.0%, which was directly used in the next reaction without further purification.
[0084] NMR δH(CDCl 3 )5.52 (1H, q, J=3.5Hz), 5.18 (1H, dt, J=10.0Hz, J=3.5Hz), 4.85 (1H, dd, J=6.5Hz, J=2.0Hz), 4.20~4.11 (3H, m), 4.08 (2H, d, J=4.0Hz), 3.24~3.18(2H, m), 2.79~2.73(2H, m), 1.87~1.80(2H, m), 1.55(3H, s ), 1.36(3H, s), 1.26(3H, t, J=7.5Hz), 1.09(3H, J=7.5Hz).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com