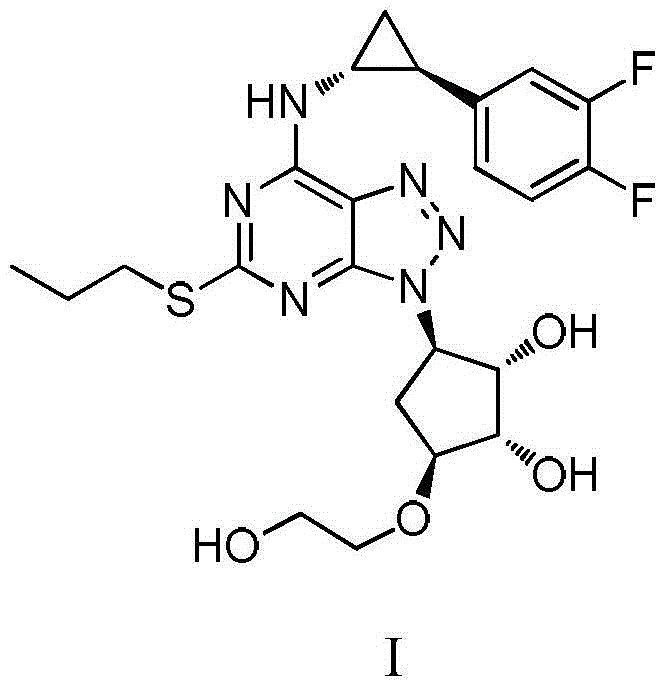
Preparation method of Ticagrelor
A technology of ticagrelor and compounds, applied in the field of preparation of anti-platelet aggregation drug ticagrelor
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1: Preparation of ticagrelor
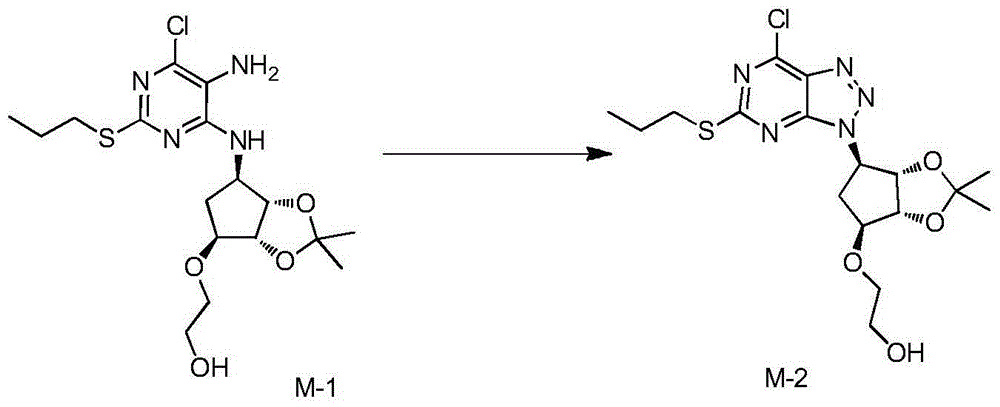
[0037] M-1 (1kg), toluene (4.31kg) and acetic acid (0.86kg) were added to the reaction flask, and sodium nitrite (0.19kg) in water (0.5kg) was added dropwise with stirring, and the temperature was controlled not to exceed 30°C. After stirring for 3 hours, a solution of potassium carbonate (1.01 kg) in water (2.01 kg) was added dropwise, after dripping, the solution was allowed to stand for liquid separation, and the upper organic phase (intermediate M-2) was stored at low temperature.
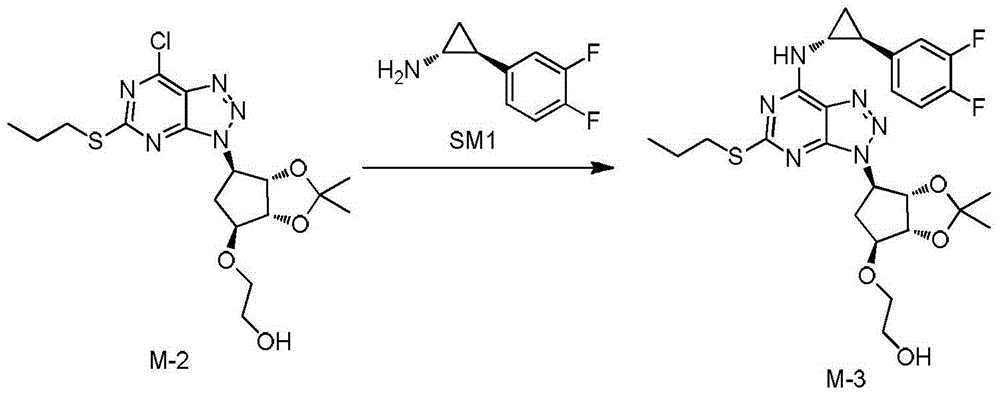
[0038] Add SM1 [0.86kg (purity 98.5%, impurity X 0.25%)] treated with 5 times the amount of acetonitrile to the water (3.24kg) solution of potassium carbonate (0.88kg) for 1 h, under nitrogen protection, pour into the previous product In the intermediate M-2 solution, the reaction was stirred at 25°C for 2 hours. Let stand for liquid separation. The organic layer is washed twice with acetic acid (0.10kg) and sodium chloride (74.8g) in water (3.13kg) sol...
Embodiment 2
[0042] Add M-1 (4.2kg), toluene (18.1kg) and acetic acid (3.61kg) into the reaction flask, add dropwise sodium nitrite (0.8kg) in water (2.1kg) solution under stirring, and control the temperature not to exceed 30℃ . After stirring for 3 hours, a solution of potassium carbonate (4.24 kg) in water (8.44 kg) was added dropwise. After the dripping, the mixture was allowed to stand for liquid separation, and the upper organic phase (Intermediate M-2) was stored at low temperature.
[0043] Add SM1 [3.61kg (purity 99.0%, impurity X 0.18%)] treated with 10 times the amount of acetonitrile to the water (13.61kg) solution of potassium carbonate (3.7kg) for 1h, and pour into the product from the previous step under nitrogen In the intermediate M-2 solution, the reaction was stirred at 22°C for 2 hours. Let stand for liquid separation. The organic layer was washed twice with acetic acid (0.42kg) and sodium chloride (0.31kg) in water (13.15kg) solution, and sodium chloride (1.3kg) in water...
Embodiment 3
[0047] Example 3: Add M-1 (6.3kg), toluene (27.2kg) and acetic acid (5.42kg) into the reaction flask, add dropwise sodium nitrite (1.2kg) in water (3.15kg) solution under stirring, and control the temperature Does not exceed 30°C. After stirring for 3 hours, a solution of potassium carbonate (6.36 kg) in water (12.66 kg) was added dropwise, after dripping, the solution was allowed to stand for liquid separation, and the upper organic phase (intermediate M-2) was stored at low temperature.
[0048] Add SM1 [5.42kg (purity 99.5%, impurity X 0.11%)] treated with 15 times the amount of acetonitrile to the water (28.74kg) solution of potassium carbonate (5.55kg) for 1 h, under nitrogen protection, pour into the product of the previous step In the intermediate M-2 solution, the reaction was stirred at 20°C for 2 hours. Let stand for liquid separation. The organic layer was washed twice with acetic acid (0.63kg) and sodium chloride (0.46kg) in water (20.42kg) solution, and sodium chlor...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com