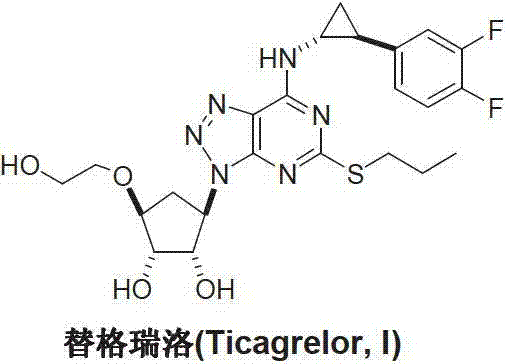
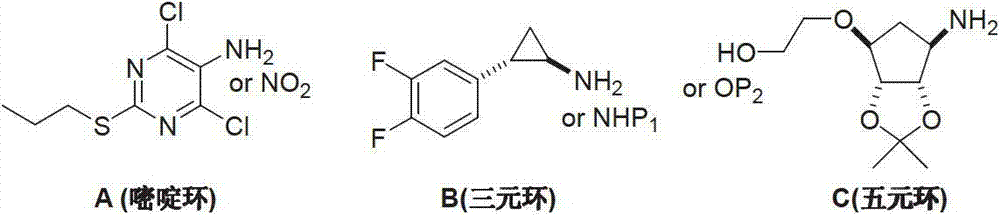
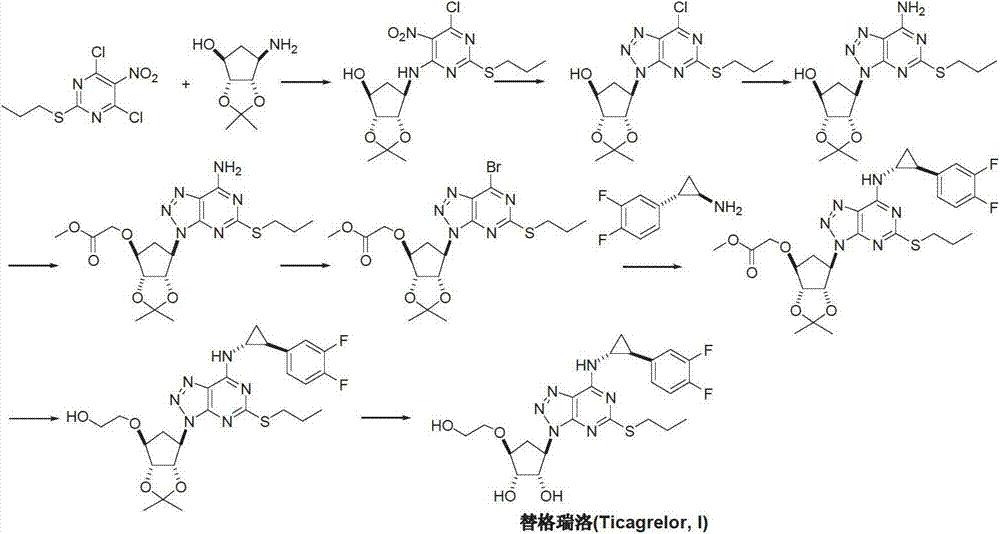
Preparation method of ticagrelor
A technology of ticagrelor and heteropurine, which is applied in the field of preparation of new anticoagulant drug ticagrelor, can solve problems such as difficult control of coupling position, avoid the use of hazardous chemicals, and the preparation process is fast, convenient and suitable. effect on large-scale industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Add 1-[3aR-(3aα, 4α, 6α, 6aα)-[[2,2-dimethyl-tetrahydro-4H-cyclopentadiene-1,3-dioxolane to the reaction flask -4-oxyl]ethanol]-6-yl]-5-amino-4-formamido-1,2,3-triazole (II) (3.27g, 10mmol), potassium carbonate (4.14g, 30mmol ), water 2mL and dichloromethane 50mL. Cool down to 0°C, slowly add a solution of carbon dichlorosulfur (III) (3.45 g, 30 mmol) in 25 mL of dichloromethane dropwise, and react for 2 hours. The reaction solution was poured into ice water to quench the reaction, and the organic phase was separated. The aqueous phase was extracted twice with dichloromethane, the combined organic phases were dried and the solvent was removed under reduced pressure. The crude product was recrystallized from ethyl acetate to obtain off-white solid 9-[3aR-(3aα, 4α, 6α, 6aα)-[[2,2-dimethyl-tetrahydro-4H-cyclopentadiene-1, 3-dioxolane-4-oxyl]ethanol]-6-yl]-2-thioxo-6-oxo-8-azapurine (IV) 3.14g, yield 85.1%.
Embodiment 2
[0043] Add 9-[3aR-(3aα, 4α, 6α, 6aα)-[[2,2-dimethyl-tetrahydro-4H-cyclopentadiene-1,3-dioxolane to the reaction flask -4-oxyl]ethanol]-6-yl]-2-thioxo-6-oxo-8-azapurine (IV) (1.85g, 5mmol), potassium hydroxide solution (0.1M, 10mL) and Acetonitrile 25mL, room temperature was added dropwise bromopropane (V) (1.53g, 12.5mmol) in acetonitrile solution. The reaction was stirred at room temperature for 15 hours. The solvent was recovered under reduced pressure, the residue was extracted 3 times with dichloromethane, the organic phases were combined, dried, and distilled under reduced pressure to obtain the oily substance 9-[3aR-(3aα, 4α, 6α, 6aα)-[[2,2-dimethyl Base-tetrahydro-4H-cyclopentadiene-1,3-dioxol-4-oxyl]ethanol]-6-yl]-2-propylmercapto-6-oxo-8-aza Purine (VI) 1.83g, yield 89.1%.
Embodiment 3
[0045] Under nitrogen protection, add 9-[3aR-(3aα, 4α, 6α, 6aα)-[[2,2-dimethyl-tetrahydro-4H-cyclopentadiene-1,3-di Oxolane-4-oxyl]ethanol]-6-yl]-2-propylmercapto-6-oxo-8-azapurine (VI) (1.95g, 5mmol), benzotriazole-1 -Oxyloxytris(dimethylamino)phosphonium hexafluorophosphate (BOP) (3.32 g, 7.5 mmol) and acetonitrile 25 mL. Under stirring, 1,8-diazabicyclo[5.4.0]-undec-7-ene (DBU) (1.14 g, 7.5 mmol) was added dropwise, and the reaction was carried out at room temperature for 12 hours. The temperature was raised to 60° C., and the reaction was continued for 12 hours. The solvent was distilled off under reduced pressure, dissolved in 100 mL of ethyl acetate, and washed with 20 mL of 2M sodium hydroxide. The organic phase was separated, dried and concentrated under reduced pressure. The residue was dissolved in 50 mL of tetrahydrofuran, and trans-(1R, 2S)-2-(3,4-difluorophenyl)cyclopropylamine (VII) (1.0 g, 6 mmol) and sodium hydride (0.16 g, 6.5 mmol) were added ), the tempe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com