Preparation method, detection method and application for ticagrelor-related substances
A kind of technology of ticagrelor and detection method, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
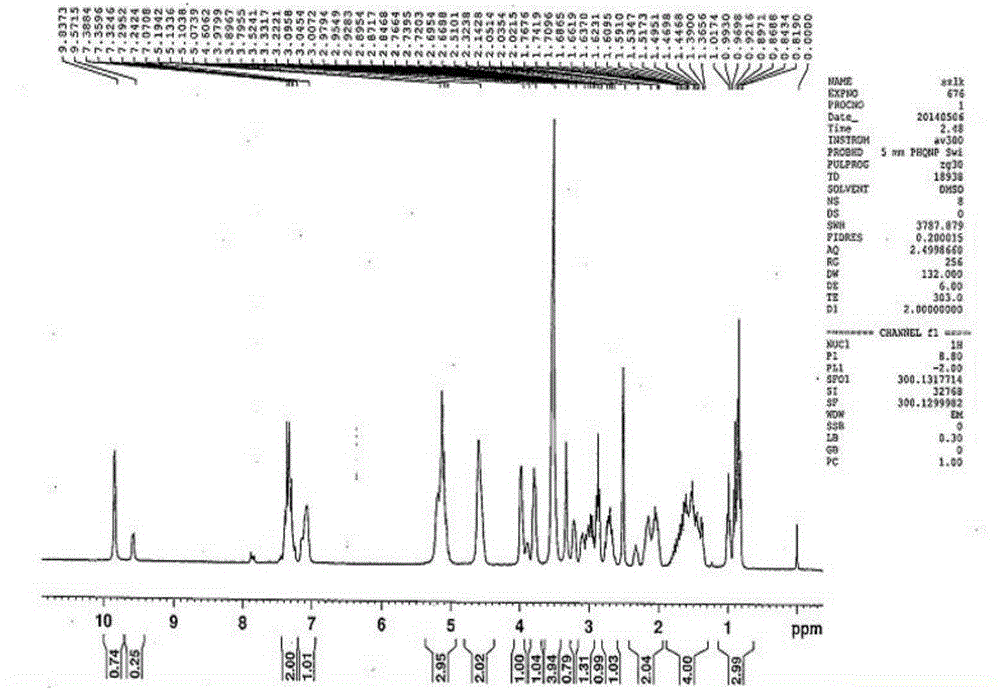
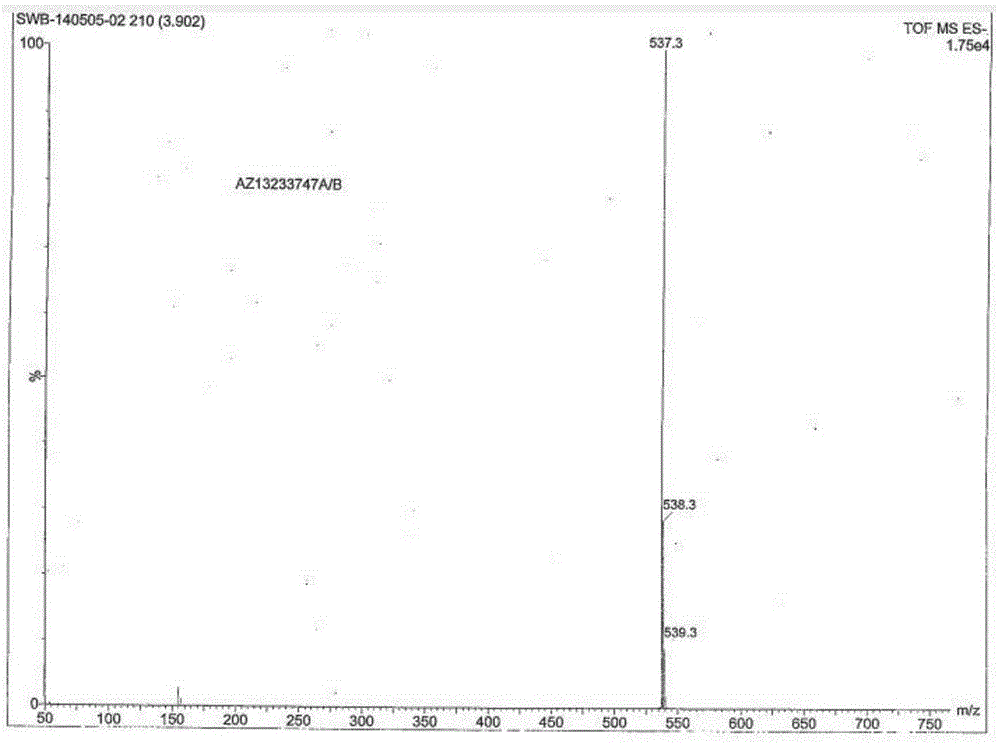
[0070] Example 1 Preparation of ticagrelor oxidized impurity compounds I and II
[0071] (1) Dissolve 15g of the intermediate in 225mL of toluene, cool in an ice-water bath to -2°C, add dropwise 210mL of toluene solution in which 18.06g of m-chloroperoxybenzoic acid is dissolved, and keep stirring at 0°C for 2.5 hours.
[0072] (2) Add 6.8g (1R, 2S)-2-(3,4-difluorophenyl) cyclopropylamine (R)-mandelate to the reaction solution, add 150mL of 0.8mol / L K 2 CO 3 The aqueous solution was stirred and reacted for 6 hours. After the reaction was completed, the layers were separated, and the toluene phase was taken for the next reaction.
[0073] (3) 120 mL of 0.3 mol / L concentrated hydrochloric acid methanol solution was added dropwise to the toluene phase. After the reaction, the mixture was allowed to stand for stratification, and the aqueous and organic phases were concentrated and evaporated to dryness respectively to obtain 7.9 g of light yellow solid and 1 white solid.
[0074...
Embodiment 2
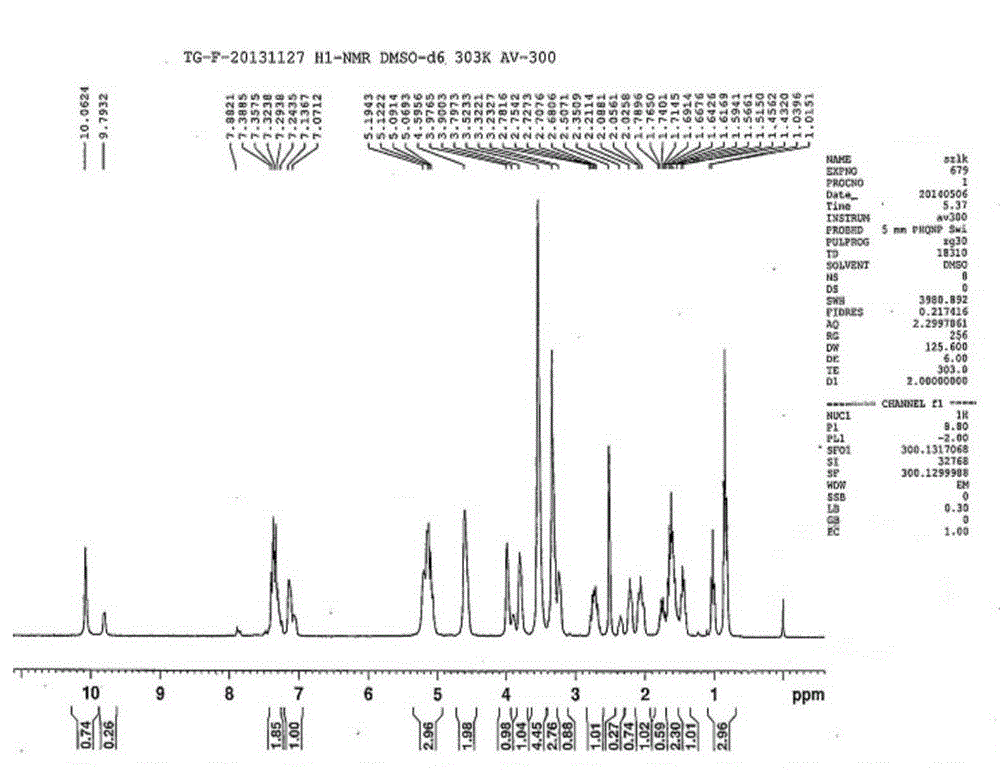
[0077] Example 2 Preparation of ticagrelor oxidized impurity compounds I and II
[0078] (1) Dissolve 15g of the intermediate in 225mL of toluene, cool in an ice-water bath to -2°C, add dropwise 320mL of toluene solution in which 30g of m-chloroperoxybenzoic acid is dissolved, and keep stirring at 0°C for 1 hour.
[0079] (2) Add 11g (1R, 2S)-2-(3,4-difluorophenyl) cyclopropylamine (R)-mandelate to the reaction liquid, add 120mL of 0.8mol / L K 2 CO 3 The aqueous solution was stirred and reacted for 6 hours. After the reaction was completed, the layers were separated, and the toluene phase was taken for the next reaction.
[0080] (3) 220 mL of 0.6 mol / L concentrated hydrochloric acid methanol solution was added dropwise to the toluene phase. After the reaction was completed, the mixture was allowed to stand for stratification, and the aqueous phase and the organic phase were concentrated and evaporated to dryness to obtain 14.9 g of light yellow solid and 1 white solid.
[00...
Embodiment 3
[0084] Example 3 Preparation of ticagrelor oxidized impurity compounds I and II
[0085] (1) Dissolve 15g of the intermediate in 225mL of toluene, cool in an ice-water bath to -2°C, add dropwise 150mL of a methanol solution in which 12g of m-chloroperoxybenzoic acid is dissolved, and keep stirring at 0°C for 5 hours.
[0086] (2) Add 5.6g (1R, 2S)-2-(3,4-difluorophenyl) cyclopropylamine (R)-mandelate to the reaction solution, add 120mL of 1mol / L K 2 CO 3 The aqueous solution was stirred and reacted for 6 hours. After the reaction was completed, the layers were separated, and the toluene phase was taken for the next reaction.
[0087] (3) 80 mL of 0.5 mol / L methanol solution of concentrated hydrochloric acid was added dropwise to the toluene phase. After the reaction was completed, the mixture was allowed to stand for stratification, and the aqueous phase and the organic phase were concentrated and evaporated to dryness respectively to obtain 4.02 g of light yellow solid and 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com