Ticagrelor pharmaceutical composition and preparation method thereof
A technology of ticagrelor and its mixture, which is applied in the field of ticagrelor oral pharmaceutical composition and its preparation, and can solve the problems of increasing safety risks and degradation impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0124] Preparation of ticagrelor crystal form A
[0125] Under stirring, dissolve 2g of ticagrelor in 3ml of N,N-dimethylformamide at 25-30°C, and then add dropwise to 30ml of methyl tert-butyl ether cooled to 0-10°C. Continue to stir and crystallize at this temperature for 2 hours, filter, wash the filter cake with n-hexane, then dry under reduced pressure at 40-45°C for 6 hours, and then dry under reduced pressure at 80-85°C to obtain ticagrelor crystal form A, 1.71g.
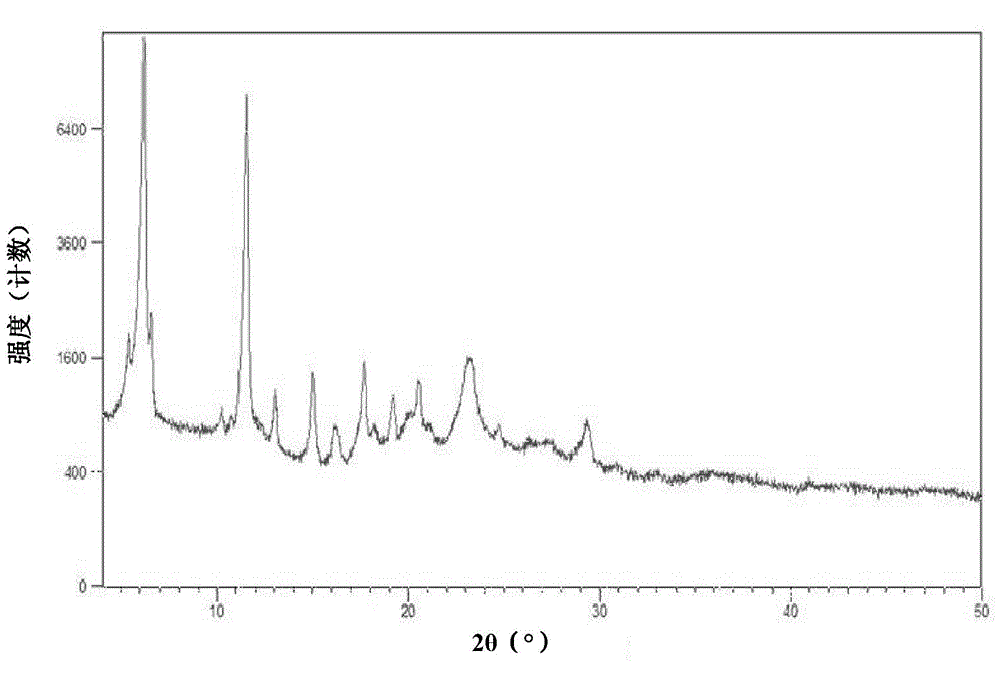
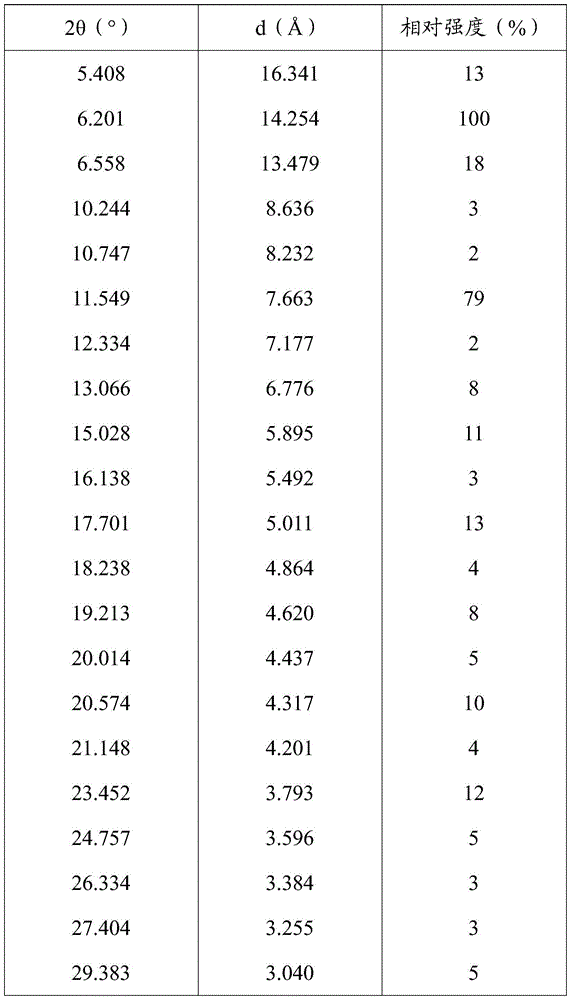
[0126] The measured X-ray powder diffraction pattern is shown in figure 1 , The measured value is shown in the following table (take the measured value corresponding to the diffraction peak with a relative intensity greater than 1%):
[0127]
Embodiment 2
[0129] Preparation of ticagrelor crystal form A
[0130] Under stirring, dissolve 2g of ticagrelor in 5ml of N,N-dimethylformamide at 20-25°C, then add to 30ml of n-heptane, lower the temperature to 5-10°C, and continue to stir and crystallize for 5 hours After filtering, the filter cake was washed with n-heptane, then dried under reduced pressure at 50-55°C for 5 hours, and then dried under reduced pressure at 70-75°C to obtain ticagrelor crystal form A, 1.65 g. The measured X-ray powder diffraction pattern and figure 1 similar.
Embodiment 3
[0132] Ticagrelor tablets and preparation method thereof
[0133] Composition of prescription:
[0134]
[0135] Preparation:
[0136] 1. Dry the microcrystalline cellulose, mannitol, sodium carboxymethyl starch and magnesium stearate in Table 3 above at about 85°C for 12 hours, and set aside.
[0137] 2. Add the sodium carboxymethyl starch and mannitol twice in equal amounts, mix for 10 minutes, and rotate at 10 rpm to obtain mixture (I).
[0138] 3. Mix ticagrelor crystal form A (D90 less than 100μm) with the above mixture (I) for 10 minutes at a speed of 10 revolutions per minute to obtain mixture (II).
[0139] 4. Mix the above mixture (II) and microcrystalline cellulose for 10 minutes at a speed of 10 revolutions per minute to obtain a mixture (III).
[0140] 5. Add magnesium stearate to the above mixture (III) and mix for 10 minutes at a speed of 10 rpm to obtain mixture (IV).
[0141] 6. Press the mixture (IV) into tablets, and control the tablet weight within ±5% of the average ta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com