6-hydroxy-8-chloro ethyl caprylate preparation method thereof
A technology of ethyl chlorooctanoate and hydroxyl, which is applied in the field of boron-containing wastewater treatment and the preparation of ethyl 6-hydroxy-8 chlorooctanoate, achieving the effects of simple synthesis steps, avoiding direct discharge, and reducing the use of organic solvents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
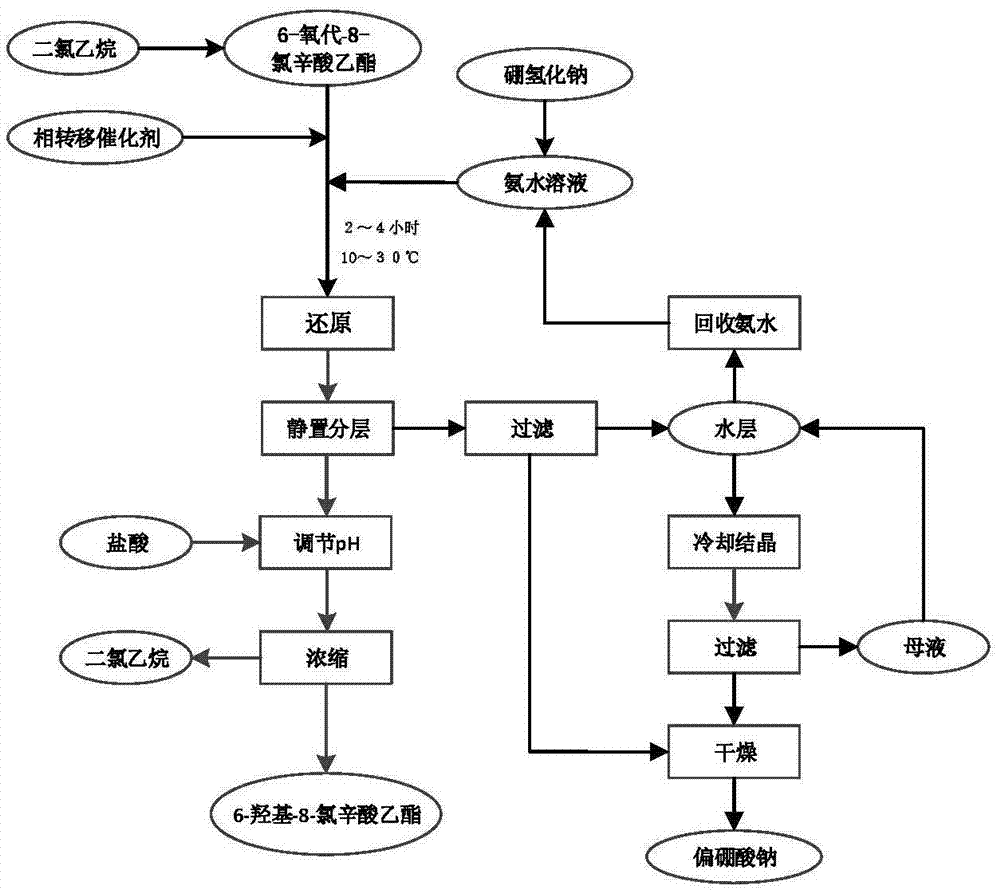
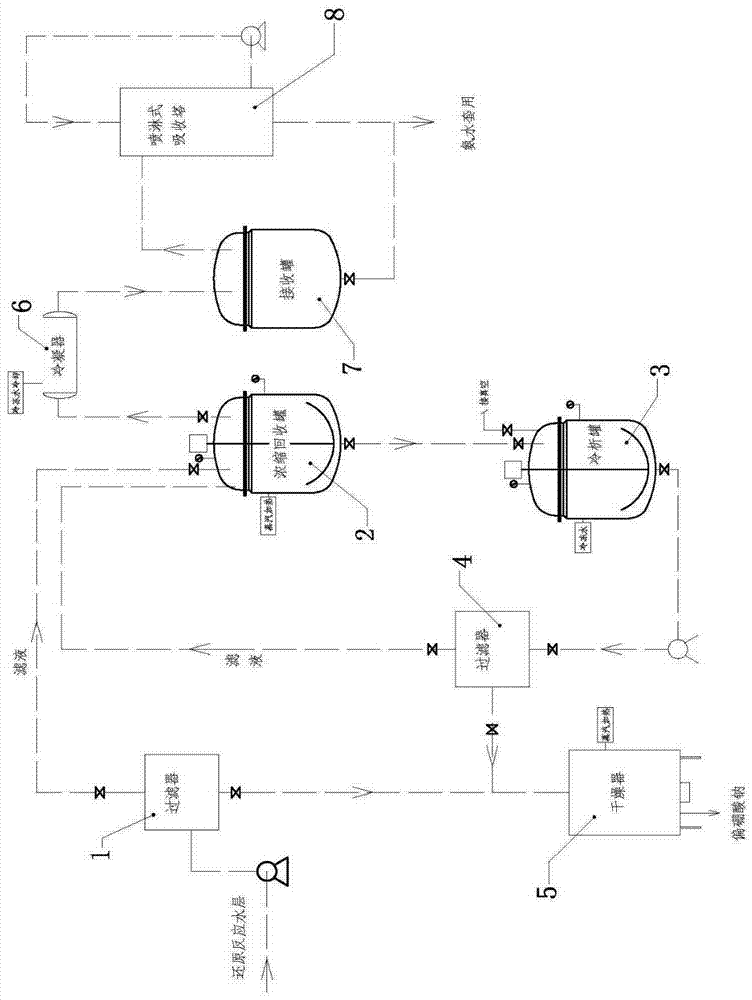
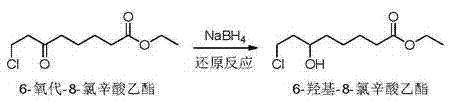
[0038] Dissolve 100Kg ethyl 6-oxo-8-chlorooctanoate in 500Kg dichloroethane, keep the temperature at 10-30°C, add 1Kg tetrabutylammonium bromide, add dropwise 17% sodium borohydride containing 5Kg Ammonia solution 100Kg, react for 4 hours, filter and separate layers, adjust the pH of the organic layer to 5-6 with hydrochloric acid, heat and concentrate under reduced pressure at 0.1MPa until the temperature is 110°C, and obtain 6-hydroxy-8-chlorooctanoic acid ethyl alcohol with a purity of 92%. Ester concentrate 98Kg. The water layer passes through the filter to obtain the precipitated sodium metaborate, the filtrate is concentrated and recovered 75Kg of 10% ammonia water for reuse, cooled and crystallized, filtered to obtain sodium metaborate, the mother liquor is returned to the sleeve, and the obtained sodium metaborate is combined to 13.8Kg, and dried at 100°C to obtain 7.6Kg, 95% purity.
Embodiment 2
[0040] Dissolve 100Kg ethyl 6-oxo-8-chlorooctanoate in 500Kg dichloroethane, keep the temperature at 10-30°C, add 1Kg tetrabutylammonium bromide, add dropwise 10% sodium borohydride containing 8Kg Ammonia solution 100Kg, of which 75Kg ammonia water is recovered from Example 1, reacted for 2 hours, filtered and separated, the organic layer was adjusted to pH=5-6 with hydrochloric acid, concentrated under reduced pressure at 0.1MPa until the temperature was 110°C, and the purity was 90%. 99Kg of 6-hydroxy-8-chlorooctanoic acid ethyl ester concentrate. The water layer was passed through a filter to obtain the precipitated sodium metaborate, the filtrate was combined with the mother liquor of Example 1, concentrated and recovered 60Kg of 7% ammonia water, cooled and crystallized, filtered to obtain sodium metaborate, and the combined sodium metaborate 21.5Kg was dried at 100°C 11Kg was obtained with a purity of 98%.
Embodiment 3
[0042] Dissolve 100Kg of ethyl 6-oxo-8-chlorooctanoate in 500Kg of dichloroethane, keep the temperature at 10-30°C, add 5Kg of tetrabutylammonium bromide, add dropwise 5% of sodium borohydride containing 8Kg Ammonia solution 100Kg, react for 4 hours, filter and separate layers, adjust the pH of the organic layer to 5-6 with hydrochloric acid, heat and concentrate under reduced pressure at 0.1MPa until the temperature is 110°C, and obtain 6-hydroxy-8-chlorooctanoic acid ethyl alcohol with a purity of 94%. Ester concentrate 95Kg. The water layer was passed through the filter to obtain the precipitated sodium metaborate, and the filtrate recovered 50Kg of 4% ammonia water. At the same time, the mother liquor was concentrated, cooled and crystallized, and then filtered to obtain sodium metaborate. The sodium metaborate was combined to obtain 20.7Kg, and dried at 100°C to obtain 11.1Kg. The purity is 94%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com