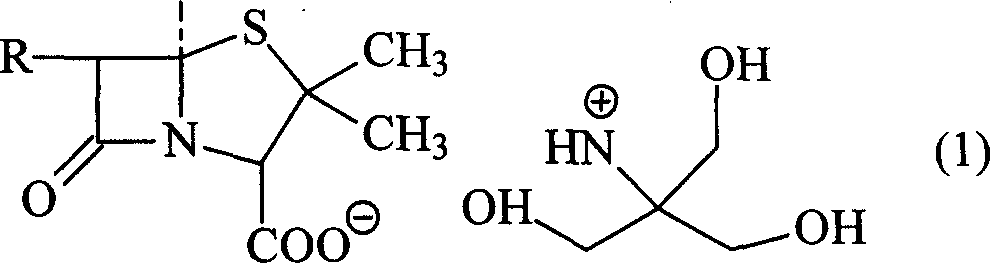
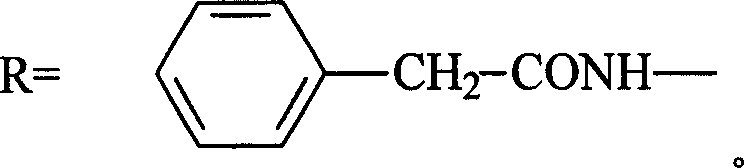
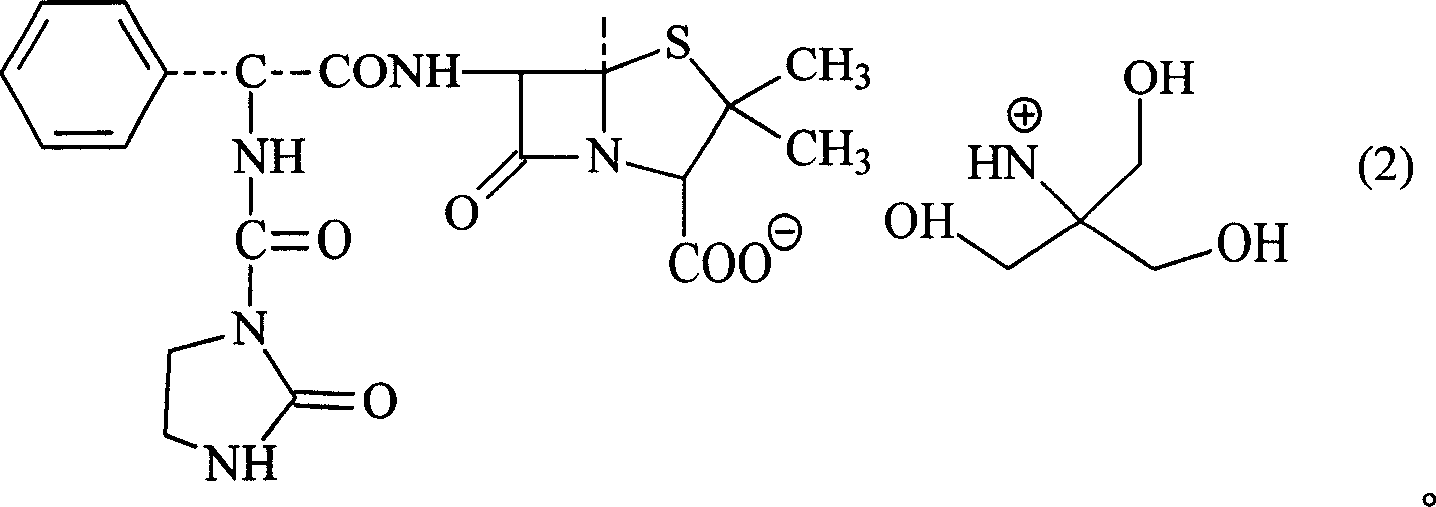
Trometamol salt in compound of cillin category, and preparation method
A technology of cillin tromethamine salt and azlocillin tromethamine salt, which is applied in the field of derivatives of cillin antibiotic compounds and their preparation, and can solve problems such as incompatibility, hypernatremia, and high risk
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Embodiment 1: the preparation of azlocillin trometamol
[0045] In a 250ml three-necked flask, add 12.1g (0.1mol) of tromethamine and 20ml of 60% ethanol, and drop into 20ml of 80% ethanol solution containing 46.2g (0.1mol) of azlocillinic acid under high temperature stirring, and stir until The mixture was clarified, stirred at room temperature for 2 hours, and the mixed reactants were dropped into 300ml (5-10°C) acetone solution to obtain white powdery crystals. The solid powder was filtered with suction, and the solid was washed with a small amount of acetone. Vacuum drying under the condition of phosphorus oxide for 24 hours yielded 51.3 g of powdery solid with a yield of 88.0%. Content analysis: (HPLC method) contained 79.0-80.8% of azlocillinic acid. The product was analyzed by elements (theoretical value: C49.4% H6.0% N14.4% O24.7% S5.5%; measured value: C49.6% H5.8% N14.7% O24.5% S5. 4%), NMR, MS, UV, IR and HPLC analysis, the product is azlocillin tromethamine...
Embodiment 2
[0046] Embodiment 2: the preparation of amoxicillin tromethamine
[0047] In a 250ml three-necked flask, add 12.1g (0.1mol) of tromethamine, 4ml of methanol, and 2ml of water, stir at high temperature, add 33.3g (0.1mol) of amoxicillin, stir until it is completely dissolved, and then stir for 2 hours. Dropped into 500ml of acetone at high temperature, a large amount of white crystalline powder was precipitated, the solid was filtered by suction, washed with a small amount of acetone, and dried at high temperature under phosphorus pentoxide for 24 hours to obtain 29.8g of white crystalline amoxicillin tromethamine. Yield: 89.5%, content analysis (HPLC method): containing 72.6-74.0% of amoxicillin acid. The product was analyzed by elements (theoretical value: C52.9% H6.6% N11.9% O21.1% S7.0%; measured value: C52.7% H6.5% N12.2% O21.3% S6. 8%), NMR, MS, UV, IR and HPLC analysis, the product is amoxicillin tromethamine salt, and the purity is 99.4% in terms of amoxicillin.
Embodiment 3
[0048] Embodiment 3: the preparation of ampicillin tromethamine
[0049] In a 250ml three-necked flask, add 12.1g (0.1mol) of tromethamine, 4ml of methanol, and 2ml of water, stir at high temperature, add 34.9g (0.1mol) of ampicillin, stir to completely dissolve and then stir for 1 hour, put the mixture in Drop it into 500ml of acetone at high temperature to precipitate a large amount of white crystalline powder, filter the solid with suction, wash with a small amount of acetone, and dry at high temperature under phosphorus pentoxide for 24 hours to obtain 41.7g of white crystalline ampicillin tromethamine salt. Yield: 88.8%, content analysis (HPLC method): containing 73.8-75.0% of ampicillin acid. The product was analyzed by elements (theoretical value: C51.1% H6.4% N11.9% O23.8% S6.8%; measured value: C51.2% H6.5% N12.0% O23.6% S6. 7%), NMR, MS, UV, IR and HPLC analysis, the product is ampicillin tromethamine salt, and the purity in terms of ampicillin is 99.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com