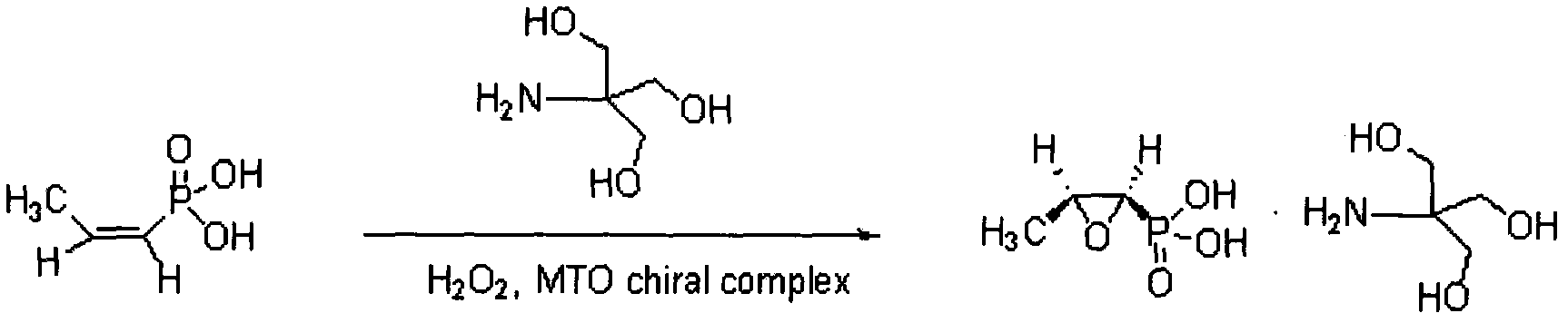
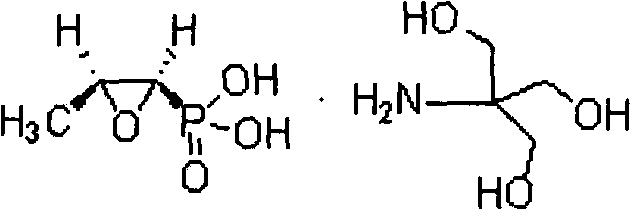New method for synthesizing fosfomycin trometamol
A technology for fosfomycin tromethamine and synthesizing fosfomycin, which is applied in chemical instruments and methods, preparation of organic compounds, preparation of aminohydroxy compounds, etc., can solve the problem of time-consuming, high cost of raw materials, and four impurities in finished products exceeding the standard And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] 1. Synthesis of Chiral Ligands
[0018] Dissolve 1.06 g of chiral bispiperidine derivatives in 10 mL of methanol, slowly add 2.49 g of methyl rhenium trioxide dropwise under stirring, and stir overnight at room temperature under nitrogen protection. The pH was adjusted to 4 with 2M methanolic hydrochloric acid, and a large amount of off-white solid was precipitated. After washing with methanol, dry in vacuum at room temperature before use.
[0019] 2. Preparation of Crude Fosfomycin Tromethamine
[0020] Take 1.22g of cis-acrylphosphoric acid and 5mL of absolute ethanol and add it to a 50mL three-necked bottle, stir to completely dissolve. Add 23 mg of the chiral ligand prepared above, and then add 1.70 g of 30% hydrogen peroxide, heat to 40° C. until completely dissolved, and react for 1 hour, and monitor the reaction by high performance liquid phase. After the reaction was completed, it was cooled, and the insoluble matter was filtered off. Under stirring, 1.37g o...
Embodiment 2
[0024] 1. Synthesis of Chiral Ligands
[0025] Dissolve 1.06 g of chiral bispiperidine derivatives in 10 mL of methanol, slowly add 2.49 g of methyl rhenium trioxide dropwise under stirring, and stir overnight at room temperature under nitrogen protection. The pH was adjusted to 4 with 2M methanolic hydrochloric acid, and a large amount of off-white solid was precipitated. After washing with methanol, dry in vacuum at room temperature before use.
[0026] 2. Preparation of Crude Fosfomycin Tromethamine
[0027] Take 1.22g of cis-acrylphosphoric acid and 5mL of absolute ethanol and add it to a 50mL three-necked bottle, stir to completely dissolve. Add 25 mg of the chiral ligand prepared above, and then add 1.80 g of 30% hydrogen peroxide, heat to 40° C. until completely dissolved, and react for 1 hour, and monitor the reaction by high performance liquid phase. After the reaction was completed, it was cooled, and the insoluble matter was filtered off. Under stirring, add 1.3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com