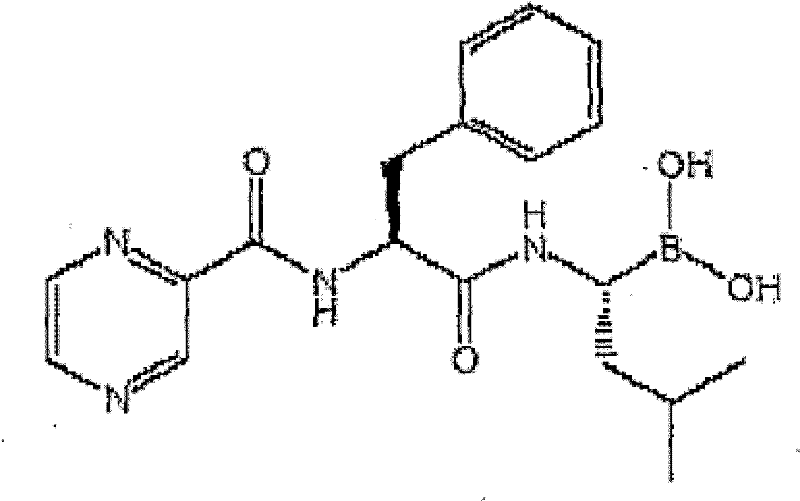
pharmaceutical composition
A composition and drug technology, applied in the directions of drug combination, drug delivery, boron compound active ingredients, etc., can solve the problems of bortezomib degradation and complex research on prefabrication
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment I
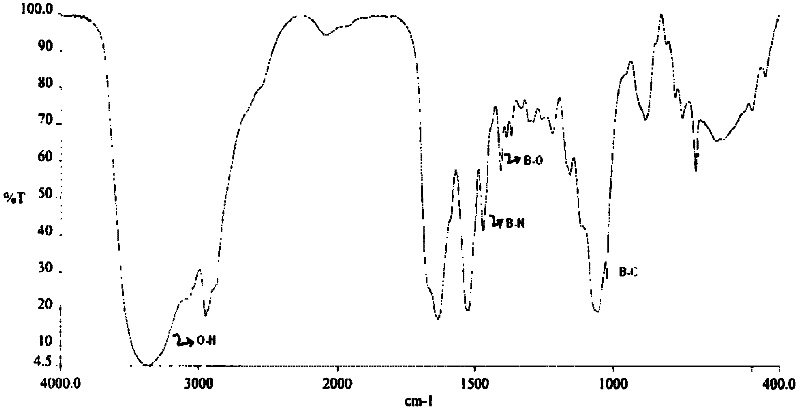
[0057] Take 3.5 mg of bortezomib containing 2.75% of total impurities. Weigh 3.31 mg of trometamol and mix in vial. With continuous stirring, water for injection (enough to make 1 ml) was added. The solution was heated at 45°C to 50°C to form a clear solution. The solution was cooled and 30 mg of sodium chloride was added to the cooled solution. The solution was filtered through a 0.45 micron needle filter and the pH was determined. The pH of the clear solution was found to be 8.68, which was adjusted to 8.01 with 5% hydrochloric acid. The clear solution was lyophilized. Reconstitution of the lyophilized cake takes less than 30 seconds and does not require an ultrasonic bath. After reconstitution, the solution was found to be stable with respect to particle formation for 24 hours, ie no particles were observed. The IR spectrum of the lyophilized composition was recorded. figure 1The IR spectrum is given. The IR spectrum of this formulation shows the formation of tromet...
Embodiment II
[0059] Take 3.5 mg of bortezomib containing 2.75% of total impurities. Weigh 3.31 mg of trometamol, wherein the total impurities are less than 0.51%, and the optical rotation is -53.4 °, and the impurity A is accurately weighed and put into a vial. Weigh 3.31 mg of trometamol and mix in the vial. With continuous stirring, water for injection (enough to make 1 ml) was added. The solution was heated at 45°C to 50°C to form a clear solution. The solution was cooled and 30 mg of sodium chloride was added to the cooled solution. The solution was filtered through a 0.45 micron needle filter and the pH was determined. The pH of the clear solution was found to be 8.68, which was adjusted to 8.01 with 5% hydrochloric acid. The clear solution was lyophilized. Reconstitution of the lyophilized cake takes less than 30 seconds and does not require an ultrasonic bath. After reconstitution, the solution was found to be stable with respect to particle formation for 24 hours, ie no parti...
Embodiment III
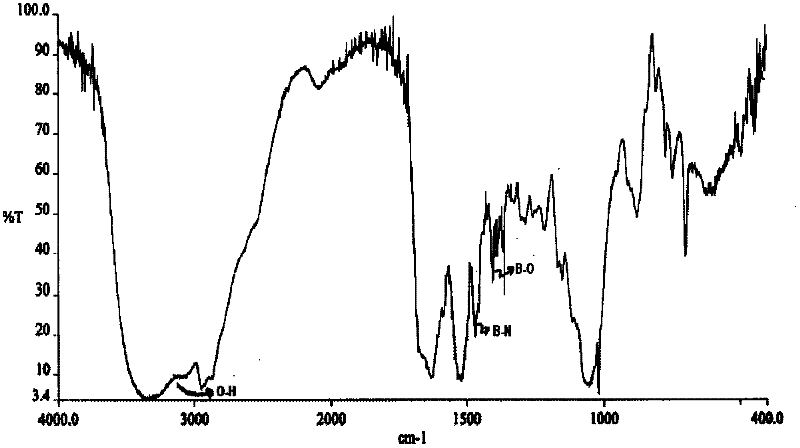
[0061] Take 3.5 mg of bortezomib containing 2.75% of total impurities. Accurately weigh 3.5 mg of bortezomib into a 5 ml vial. Weigh 3.31 mg of trometamol and mix in vial. With continuous stirring, water for injection (enough to make 1 ml) was added. The solution was heated at 45°C to 50°C to form a clear solution. The solution was filtered through a 0.45 micron needle filter and the pH was determined. The pH of the clear solution was found to be 8.75 and was adjusted to 7.89 with 5% hydrochloric acid. The clear solution was lyophilized. Reconstitution of the lyophilized cake required less than 30 seconds and did not require an ultrasonic bath. After reconstitution, the solution was found to be stable with respect to particle formation for 24 hours, ie no particles were observed. The IR spectrum of the lyophilized composition was recorded. figure 2 The IR spectrum is given. The IR spectrum of this formulation shows the formation of tromethamine salts with strong B-N b...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com