Method for synthesizing 1,2,4-butanetriol
A technology of butylene glycol and mixed solution, applied in 1 field, can solve the problems of being difficult to obtain, difficult to control the reaction temperature, and high local concentration, and achieve the effects of easy control of the reaction temperature, easy industrialization, and mild operating conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
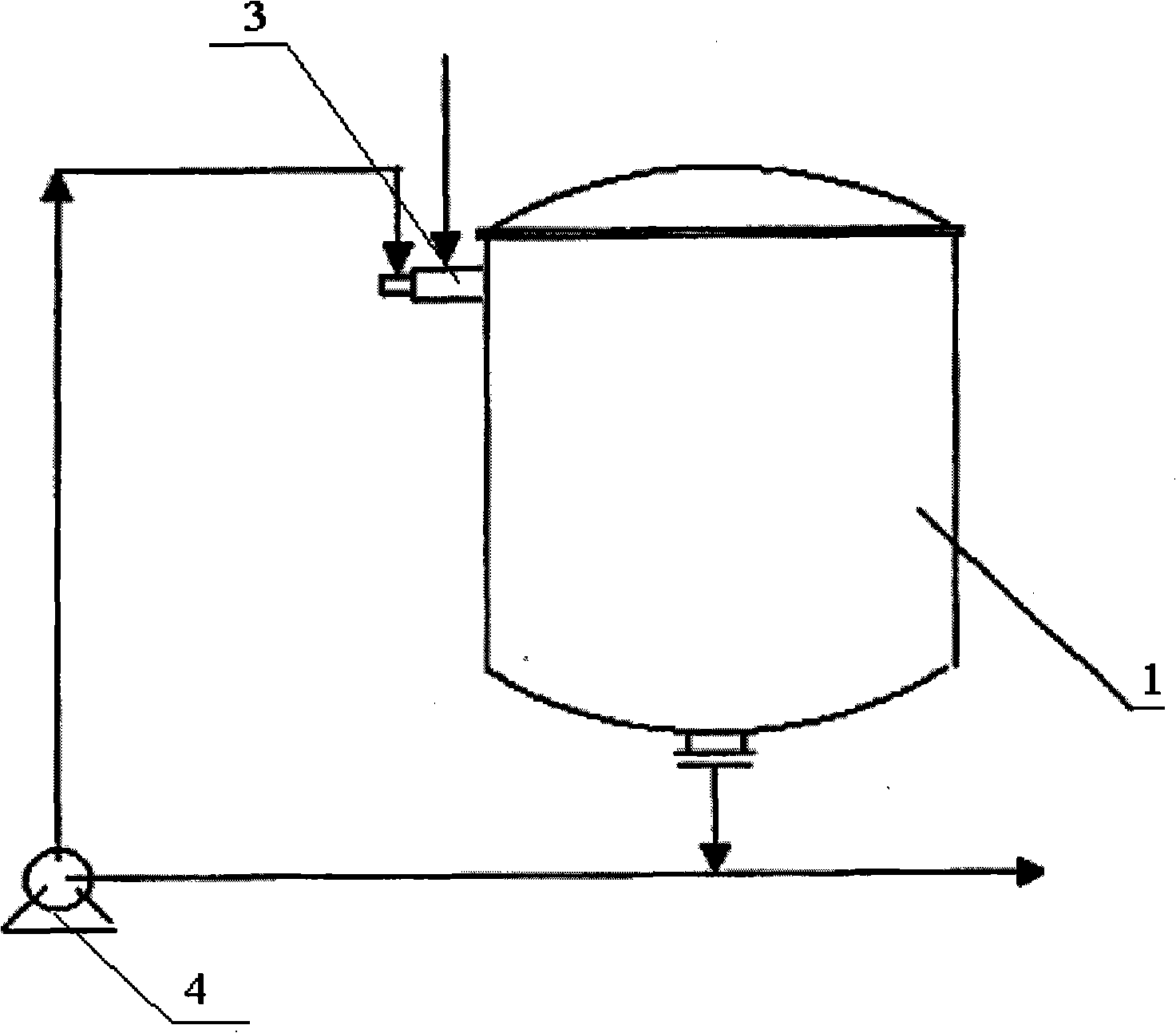
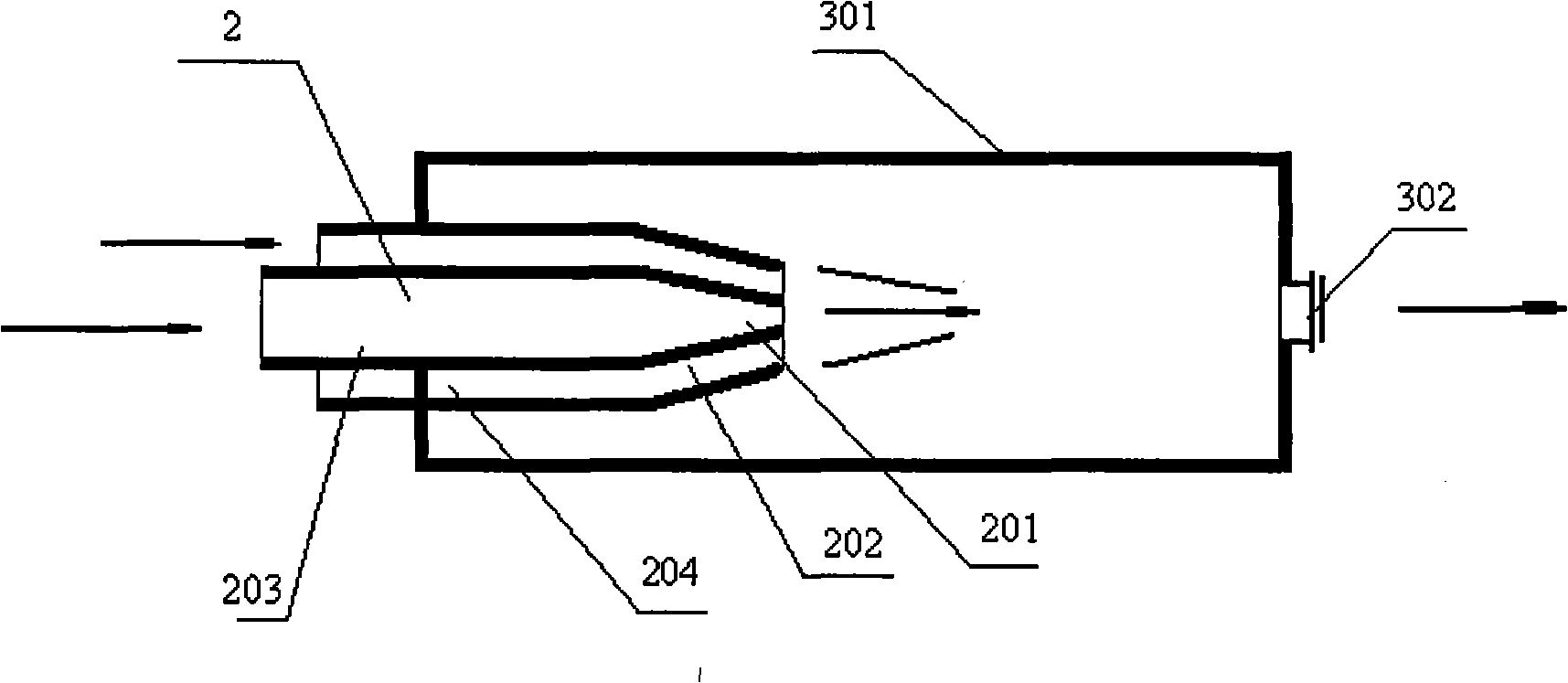
[0038] (1) With the mixed solution of 60kg butylene glycol, 400 milliliters of N-methylmorpholine and 540 grams of tungstic acid, the volume concentration is 90% oxygen-enriched gas by being arranged on the top of the reactor to be provided with the mixing of two channel nozzles After mixing in the reactor, enter the reactor to react;
[0039] The material in the reactor is sent to the mixer at the top of the reactor by a circulation pump for recycling, the reaction temperature is 25°C, the reaction time is 10 hours, and then the epoxide is collected;
[0040] The amount of oxygen-enriched gas entering the mixer of the two-channel nozzle is 1.5 times the theoretical reaction molar amount of oxygen and butylene glycol.
[0041] (2) the epoxide of 120kg step (1), 50kg volume concentration are 95% ethanol in the presence of 1.2kg catalyzer, carry out hydrogenation reaction with hydrogen, the reaction time is 1.5 hours, and temperature of reaction is 160 ℃, the reaction pressure ...
Embodiment 2
[0044] (1) With the mixed solution of 60kg butylene glycol, 400 milliliters of N-methylmorpholine and 540 grams of tungstic acid, the volume concentration is that the oxygen-enriched gas of 60% is provided with the mixing of two channel nozzles that are arranged on the top of the reactor After mixing in the reactor, enter the reactor to react;
[0045] The material in the reactor is sent to the mixer at the top of the reactor by a circulating pump for recycling, the reaction temperature is 25°C, the reaction time is 14 hours, and then the epoxide is collected;
[0046] The amount of oxygen-rich gas entering the mixer of the two-channel nozzle is 1 time of the theoretical reaction mole amount of oxygen and butylene glycol.
[0047] (2) the epoxide of 120kg step (1), 50kg volume concentration are 35% ethanol in the presence of 1kg catalyzer, carry out hydrogenation reaction with hydrogen, the reaction time is 0.5 hour, and reaction temperature is 130 ℃ , the reaction pressure i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com