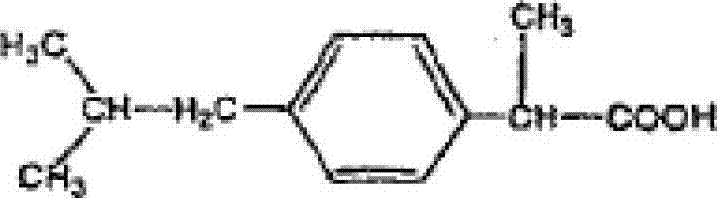
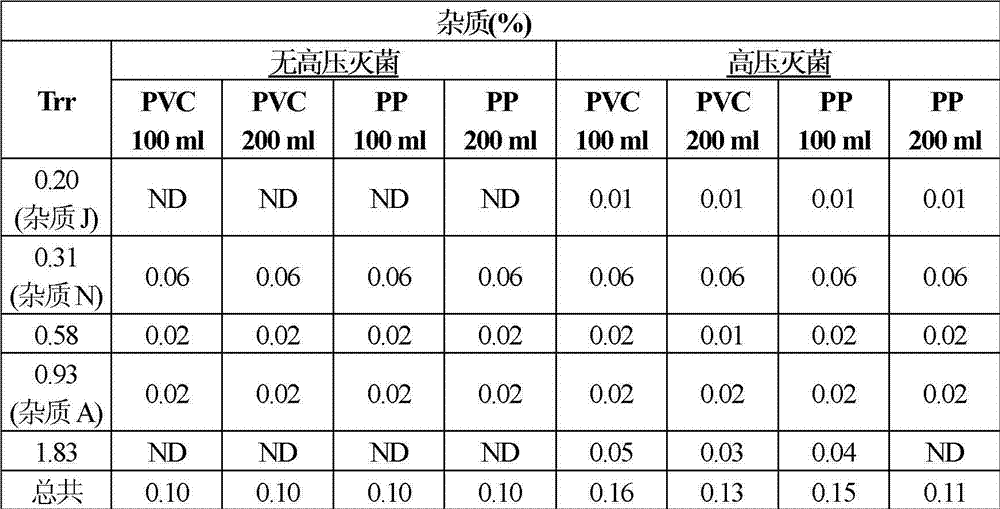
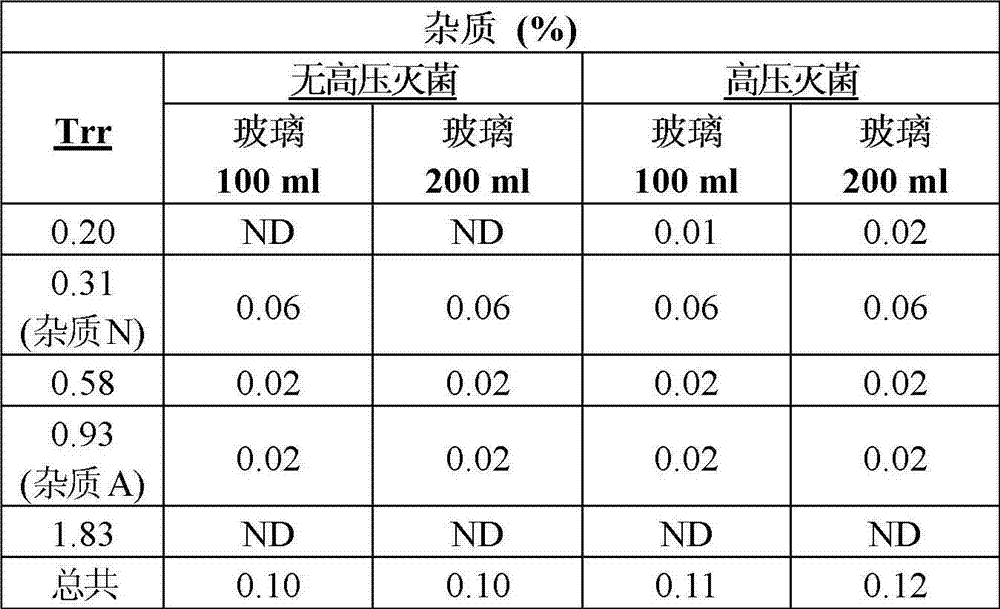
Pharmaceutical composition of ibuprofen for injection
A composition and drug technology, applied in the direction of drug combination, drug delivery, antipyretics, etc., can solve the problems of endangering the solubility of ibuprofen, no prompt, unsuitable for sterilization, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0014] Therefore, the liquid pharmaceutical composition of the present invention comprises ibuprofen at a concentration of 2-6 mg / mL and preferably about 4 mg / mL, tromethamine at a concentration of 1.8-5.8 mg / ml and preferably about 3.8 mg / ml, and The NaCl is necessary to provide suitable isotonicity, that is, usually about 300 mOsm / kg, which requires a NaCl concentration of preferably about 7.7 mg / ml. It is believed that tromethamine helps to increase the dissolution rate of ibuprofen in aqueous solvents and also helps maintain the stability of ibuprofen in solution. In the composition of the present invention, tromethamine is added at a concentration of 1.5-5.8 mg / ml, and preferably about 3.8 mg / ml. The pH of the compositions of the present invention is 7.0-12, preferably 7.0-9.5, more preferably 7.5-9.0, even more preferably 8.0-9.0, and most preferably about 8.5, depending on the container in which they are placed. The adjustment of the pH can be carried out by any means k...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com