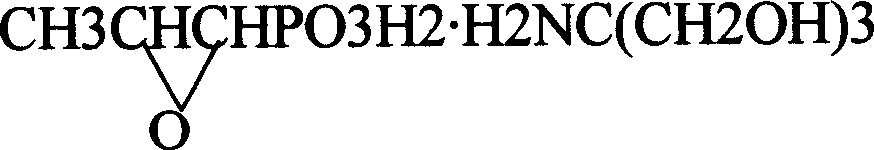
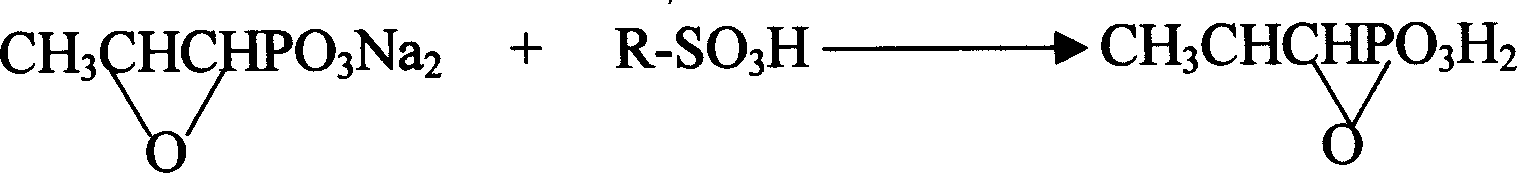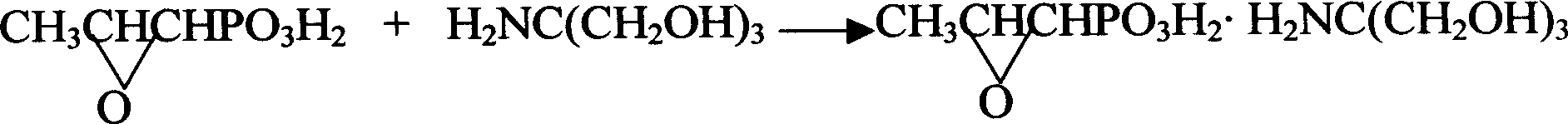Novel pulveres fosfomycin trometamol synthetic method
A technique for trometamol and tromethamine, which is applied in the field of synthesizing fosfomycin monotromethamine, can solve problems such as inappropriate rework, and achieve the effects of improving the production capacity of equipment and increasing the output of a single batch
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Measure 70ml of anhydrous methanol and add it to a beaker containing 170ml of dry D001 cation exchange resin, stir evenly, then add the resin together with methanol to the exchange column with interlayer, open the low temperature bath, and lower the column temperature to - 20°C. Adjust the methanol liquid level in the column just slightly higher than the resin level, then add 14.5g of fosfomycin sodium (0.65% glycol) into the exchange column to start the exchange reaction, use anhydrous methanol as eluent, and continuously elute until there is no more fosfomycin acid flowing out from the bottom of the column, use about 600ml of anhydrous methanol, and directly add the eluted fosfomycin acid methanol solution into a 1000ml four-necked bottle filled with 8.6g tromethamine , carry out neutralization reaction, then add a small amount of activated carbon for decolorization for 10 minutes, filter, concentrate under reduced pressure to a liquid volume of 40-50ml, add 80ml of a...
Embodiment 2
[0032] Measure 100ml of anhydrous methanol into a beaker containing 220ml of dry D001 type cation exchange resin, stir evenly, then add the resin together with methanol into the exchange column with interlayer, open the low temperature bath, and lower the column temperature to - 17°C. Adjust the methanol liquid level in the column to be just slightly higher than the resin level, then add 14.5g of neutral fosfomycin sodium into the exchange column to start the exchange reaction, use anhydrous methanol as the eluent, and continue to elute until the bottom of the column is no longer Until fosfomycin acid flows out again, use about 650ml of anhydrous methanol to directly add the eluted fosfomycin acid methanol solution into a 1000ml four-neck bottle containing 26.0g fosfomycin bis tromethamine salt. Perform neutralization reaction, then add a small amount of activated carbon for decolorization for 10 minutes, filter, concentrate under reduced pressure to a liquid volume of 50-60ml...
Embodiment 3
[0036] Measure 50ml of anhydrous methanol and add it to a beaker containing 150ml of dry D001 cation exchange resin, stir evenly, then add the resin together with methanol to the exchange column with interlayer, open the low temperature bath, and lower the column temperature to - 14°C. Adjust the methanol liquid level in the column to be just slightly higher than the resin level, then add 14.5g of fosfomycin sodium (1.0% glycol) into the exchange column to start the exchange reaction, use anhydrous methanol as eluent, and continuously elute , until no more fosfomycin acid flows out from the bottom of the column, use about 600ml of anhydrous methanol, and directly add the eluted fosfomycin acid methanol solution to 1000ml of fosfomycin bis tromethamine salt containing 28g In a four-neck flask, carry out neutralization reaction, then add a small amount of activated carbon for decolorization for 10 minutes, filter, concentrate under reduced pressure to a liquid volume of 50-60ml,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com