Swine fibrin glue powder inhalation and preparation method and application thereof
A technology of fibrin glue and fibrin, applied in the field of biomedicine, can solve the problems such as hindering the application of fibrin sealant, difficult to control the configuration time, prolonging the waiting time for surgery, etc., and achieves the effects of optimal sealing, stable medicine, and convenient carrying and storage.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
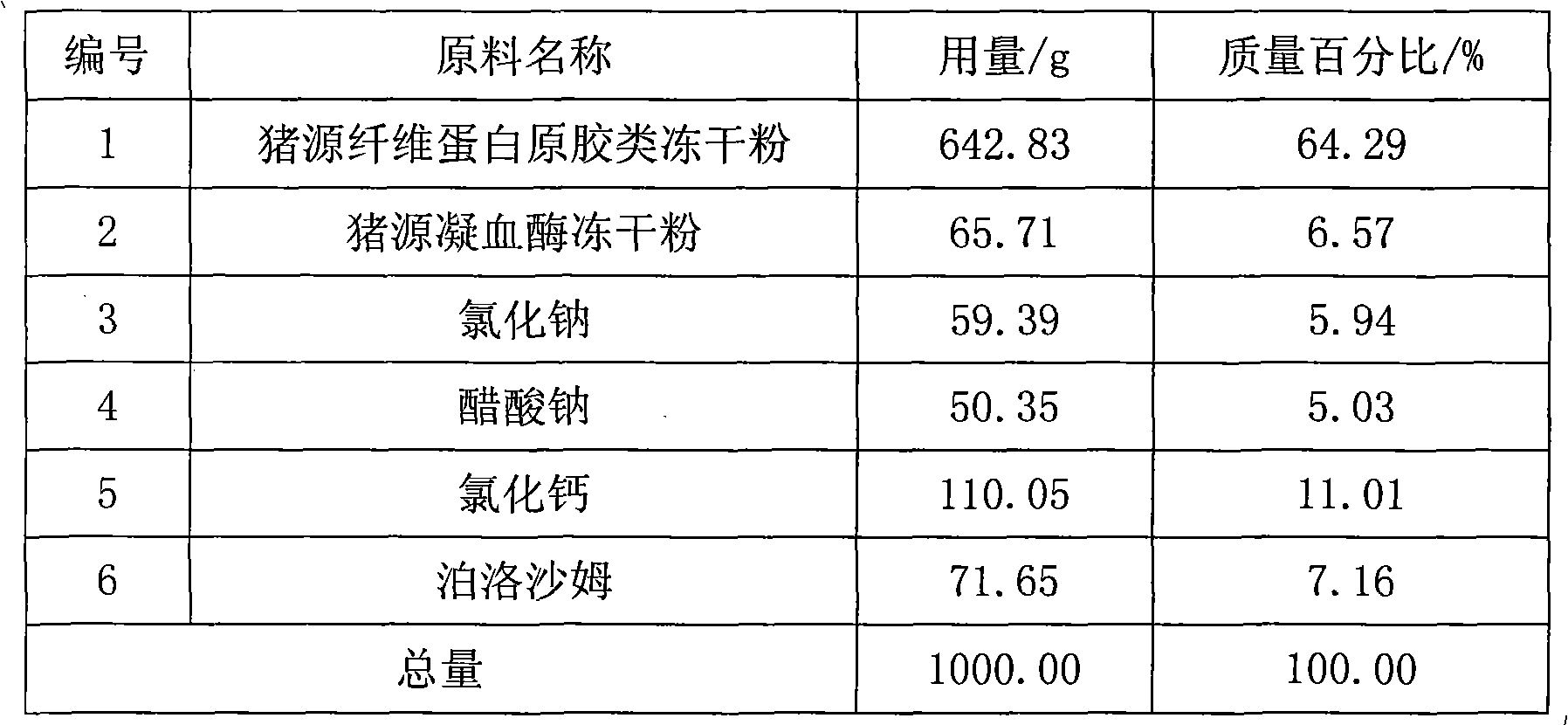
[0034] The preparation method of the above-mentioned porcine source fibrin gel powder spray comprises the following steps: (1) preparing porcine source fibrinogen gum freeze-dried powder, porcine source thrombin freeze-dried powder, sodium chloride, sodium acetate, calcium chloride , trishydroxymethylaminomethane, physiologically acceptable antistatic agent, and weighed according to formula; Grinding, the particle size of the pulverized powder is 75 μm to 180 μm; (3) mixing sodium chloride and / or sodium acetate, calcium chloride and / or trishydroxymethylaminomethane, and a physiologically acceptable antistatic agent, Then adopt the jet milling method to pulverize, and the particle size of the pulverized mixture is 90 μm to 250 μm; (4) mix the above pulverized components in a fluidized state mixing mode to form porcine-derived fibrin glue micropowder; (5) Filled in the powder mist injection device.
[0035] Commonly used micronization methods include jet milling, ball milling, ...
Embodiment 1
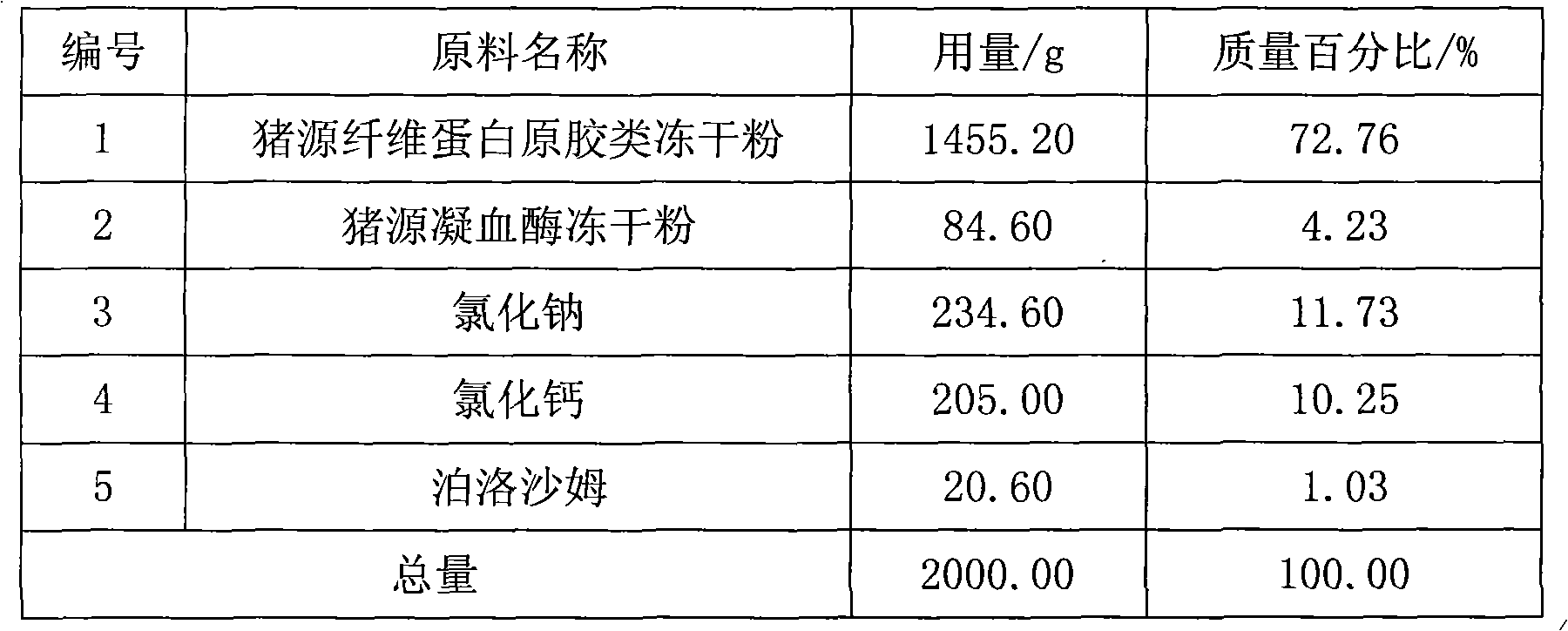
[0038] 1. Preparation of pig-derived fibrinogen gum freeze-dried powder: collect 55600ml of pig blood, add 6000ml of anticoagulant containing 0.147mol / L sodium citrate and 0.154mol / L sodium chloride, and store in a low-temperature storage at 4°C Let it stand for 6 hours, let it separate into layers, and separate the upper layer of plasma; centrifuge the upper layer of plasma in a high-speed centrifuge at 10°C at a speed of 3500 rpm for 40 minutes, collect the plasma, and filter it with a 0.22 μm filter membrane; 20000ml of plasma, add 73.5g of glycine to dissolve it, cool the plasma to 4°C, add 2605.4g of sodium acetate to dissolve it, stir for 30 minutes, centrifuge at 3500 rpm for 40 minutes in a high-speed refrigerated centrifuge at 10°C, The precipitate was collected to obtain 2520.8 g of precipitate. Crush the collected precipitate, add 33 liters of buffer solution with a pH value of 7.5 made of sodium citrate and trishydroxymethylaminomethane, stir until completely disso...
Embodiment 2
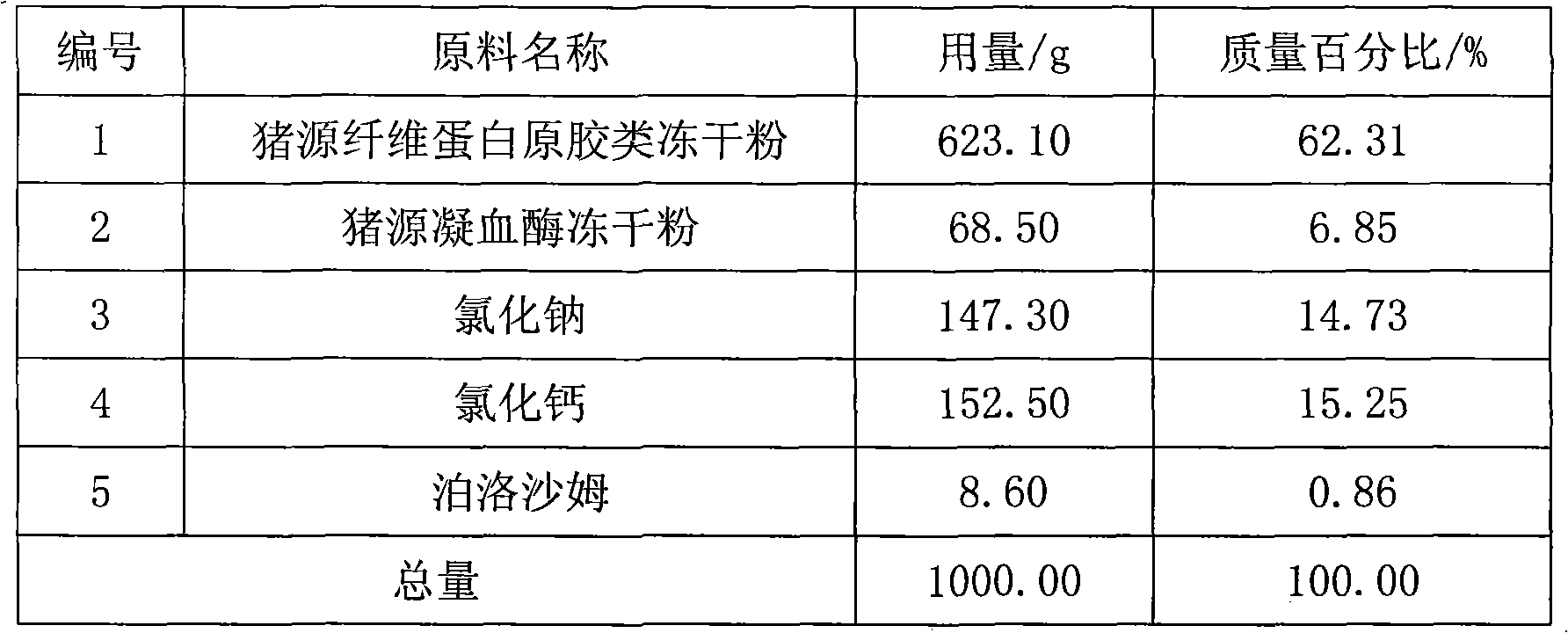
[0044]1. Pig-derived fibrinogen gum freeze-dried powder: Collect 60,000ml of pig blood, add 6,000ml of anticoagulant containing 0.145mol / L sodium citrate and 0.151mol / L sodium chloride, and place it in a low-temperature storage at 5°C After 6 hours, let it separate into layers, separate the upper layer of plasma, centrifuge at 10°C at 5,000 rpm for 60 minutes with a high-speed refrigerated centrifuge, collect the plasma, and filter it with a 0.22 μm filter membrane; take 22,000 ml of filtered plasma, add Glycine 95.8g was dissolved, the plasma was cooled to 3°C, sodium acetate 2931.8g was added to dissolve it, stirred for 30 minutes, and the high-speed refrigerated centrifuge was centrifuged at 6000 rpm for 30 minutes at 10°C to collect the precipitate. A precipitate of 2685.8 g was obtained. Crush the collected precipitate, add 35 liters of buffer solution with a pH value of 7.8 made of sodium citrate and trishydroxymethylaminomethane, stir until completely dissolved, filter,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com