Crude heparin sodium purification technology
A technology of heparin sodium and process, applied in the field of purification process of heparin sodium, can solve the problems of long production cycle, poor effect, increased environmental protection pressure and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
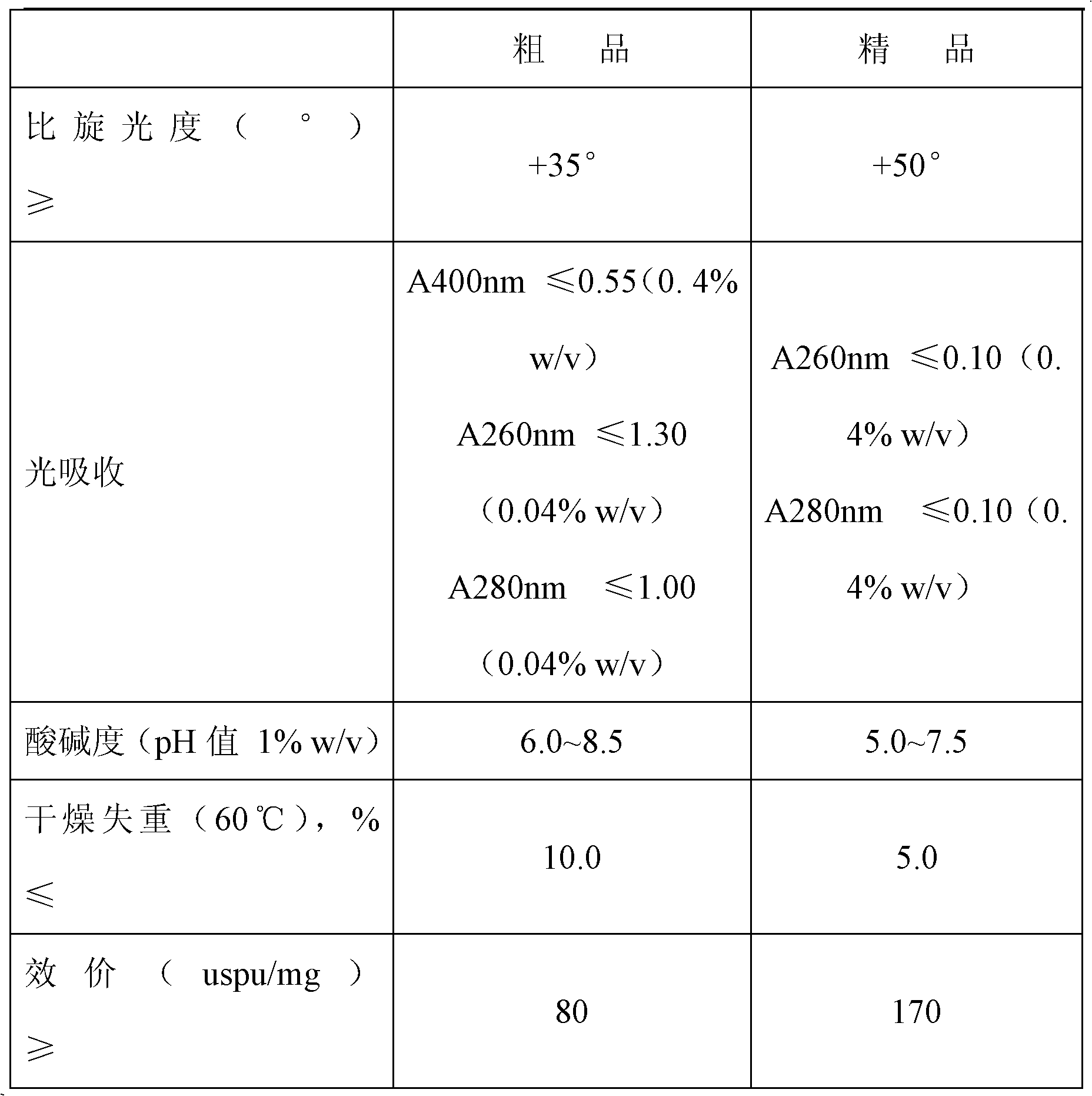
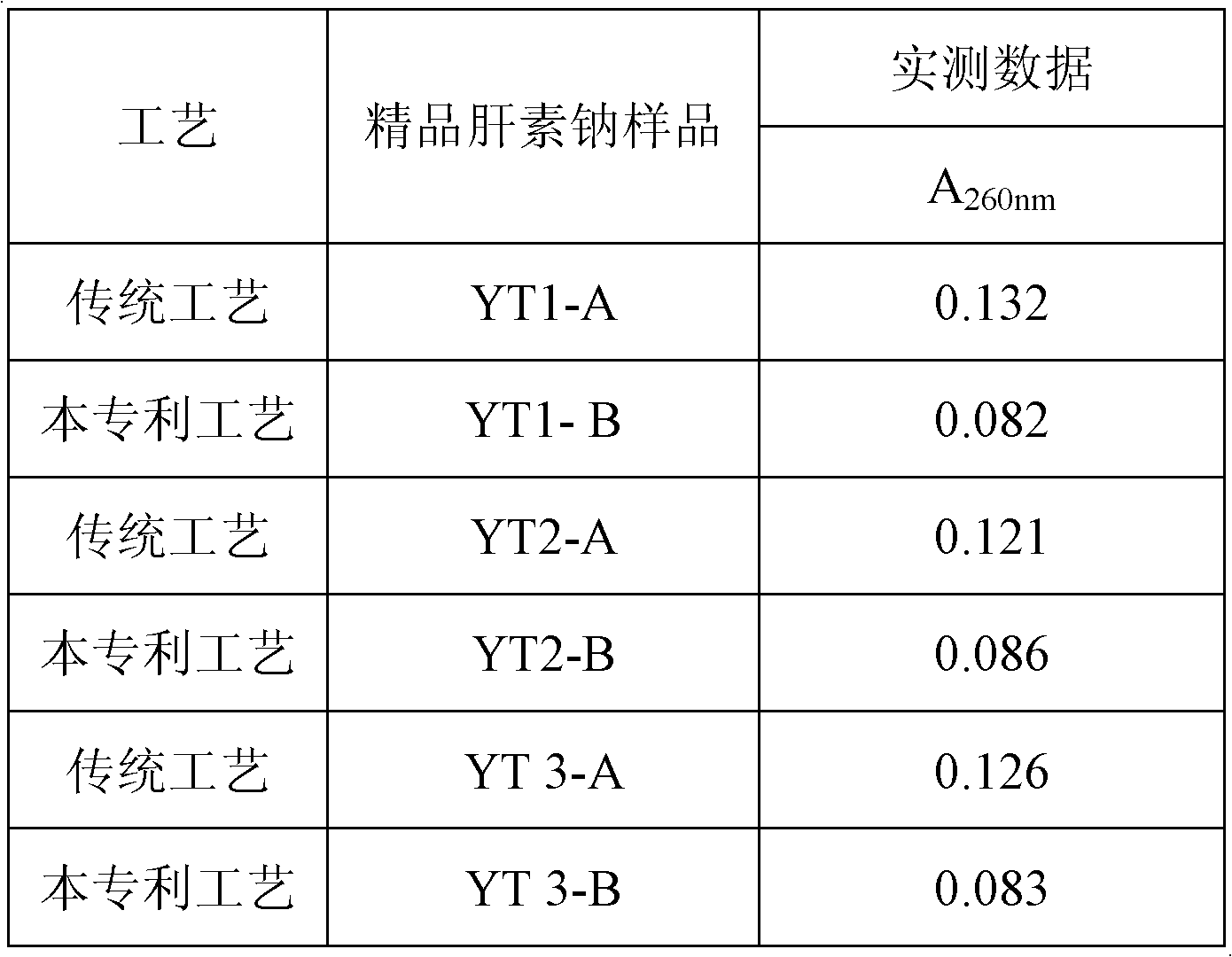
[0014] A kind of purification process of crude product heparin sodium, comprises the following steps successively: ①The crude product heparin sodium that total activity of heparin is 10000Mega uspu is dissolved in 1250 liters of 4% sodium chloride solution, adds a certain amount of alkaline protease, specifically per gram Crude heparin sodium plus 200 units of alkaline protease (alkaline protease is an active substance, which is used to describe its activity in units of potency, and the alkaline protease used is 100000u / g) was incubated at 55°C for enzymolysis; ②The obtained The temperature of the solution is raised to 90°C for inactivation, then lowered to 60°C, the pH is adjusted to 11 with alkali, diatomite is added, and the mass of diatomite is 0.02%-0.08% of the obtained solution, and insoluble impurities are discarded after high-speed centrifugation; ③ The obtained clarified liquid is cooled below 4°C, specifically between -2°C and 4°C, and polymerized aluminum silicate i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com