Preparation method of high-quality low-molecular weight dalteparin sodium
A low-molecular, high-quality technology, applied in the field of biomedicine, can solve the problems of difficult product quality, unconcentrated molecular weight distribution, excessive residual impurities, etc., and achieve good antithrombotic activity and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
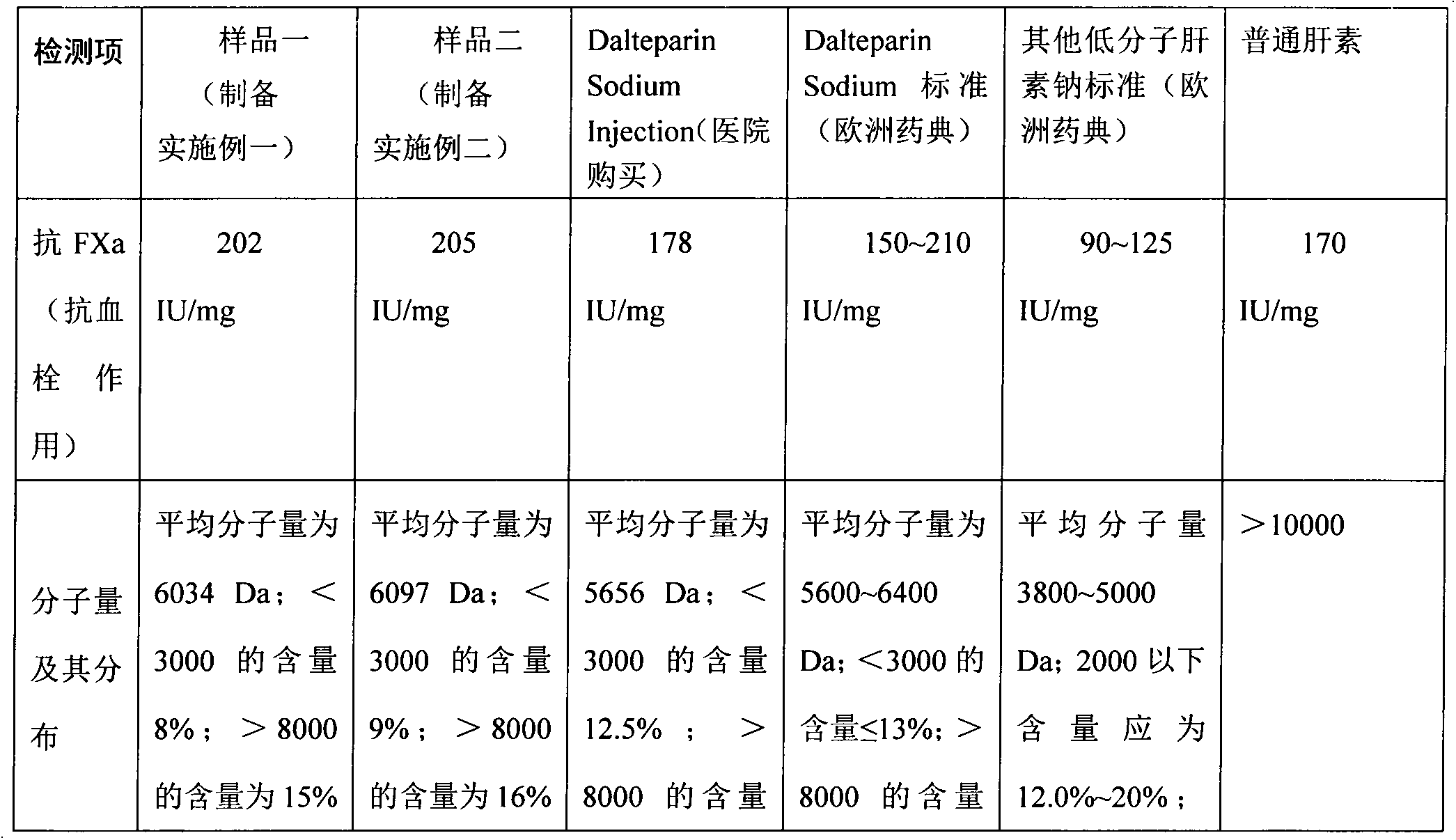
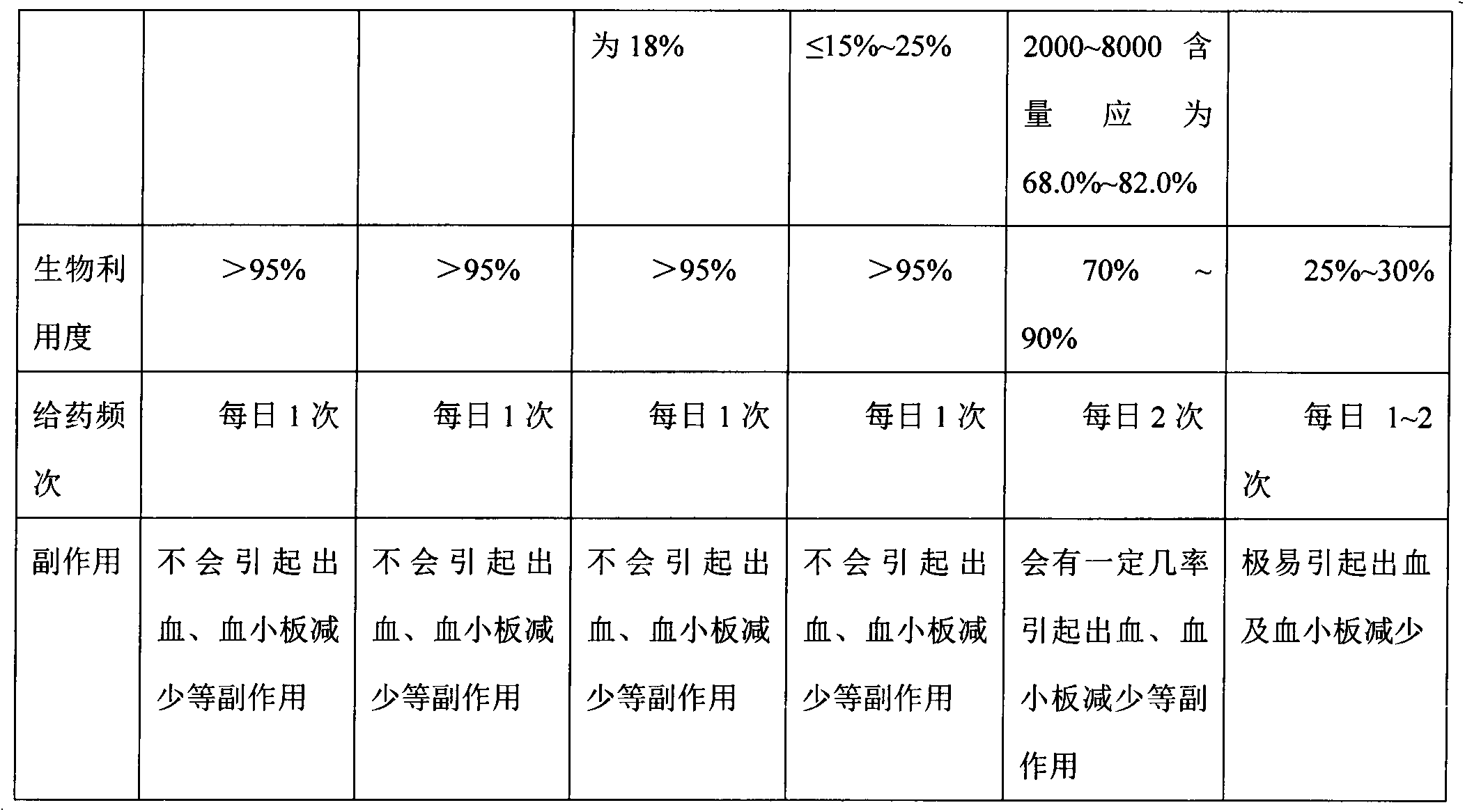
preparation Embodiment 1
[0026] Specific steps:
[0027] 1) Enzymolysis: Weigh 100 g of crude heparin sodium, add 1200 g of water to it, and dissolve to obtain a raw material solution; adjust the temperature of the raw material solution to 37° C., add 1 g of papain, 0.5 g of ribonuclease II and 0.5 g of deoxyribonuclease I respectively. 5g, stirred and reacted for 12 hours, removed the precipitate, filtered the supernatant, and collected the filtrate;
[0028] 2) Oxidation: control the temperature of the filtrate obtained in step 1) to 45° C., adjust the pH value of the filtrate to 9.5 to 10 with 20% (w / v) sodium hydroxide solution, add 12 ml of hydrogen peroxide, and stir for 7 hours to obtain a purified solution ;
[0029] 3) Ultrafiltration for impurity removal: use an ultrafiltration membrane with a molecular weight cut-off of 4,000 Da to perform tangential flow circulating ultrafiltration on the purified solution obtained in step 2), and circulate the ultrafiltration for 7 hours to collect 316 m...
preparation Embodiment 2
[0037] Specific steps:
[0038] 1) Enzymolysis: Weigh 200g of crude heparin sodium, add 2000g of water to it, and dissolve it to obtain a raw material solution; adjust the temperature of the raw material solution to 37°C, add 4g of papain, 1g of ribonuclease II and 1g of deoxyribonuclease I, respectively, and stir to react After 12 hours, the precipitate was removed, the supernatant was filtered, and the filtrate was collected;
[0039] 2) Oxidation: control the temperature of the filtrate obtained in step 1) to 45° C., adjust the pH value of the filtrate to 9.5 to 10 with 20% (w / v) sodium hydroxide solution, add 25 ml of hydrogen peroxide, and stir for 7 hours to obtain a purified solution ;
[0040] 3) Ultrafiltration for impurity removal: the purified liquid obtained in step 2) was subjected to tangential flow circulating ultrafiltration using an ultrafiltration membrane with a molecular weight cut-off of 4000 Da, and the circulating ultrafiltration was performed for 7 hou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com