Quantitative determination method for titer of recombinant insect baculovirus
A technology for quantitative determination of baculovirus, applied in measuring devices, color/spectral characteristic measurement, instruments, etc., can solve the problems of no quantitative determination of recombinant insect virus titers, etc., and achieve easy-to-master technology, short time-consuming, and simple operation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1: Cell Seeding
[0028] (1) Materials Sf9 cells were purchased from China Center for Type Culture Collection; TC-100 medium and fetal bovine serum were purchased from GIBCO; 96-well plates were purchased from NUNC.
[0029] (2) Operation method
[0030] Take the Sf9 adherent growth cells in the logarithmic growth phase, and gently pipette to resuspend the cells. Centrifuge at 1,000g (horizontal rotor) for 8min. Discard the supernatant, and resuspend the cells in TC-100 medium containing 10% (V / V) fetal bovine serum to make a cell suspension. Samples were stained with trypan blue and counted.
[0031]Dilute the cells to 3 × 10 with TC-100 medium containing 10% (V / V) fetal bovine serum 4 cells / mL, added to a 96-well cell culture plate, 100 microliters per well. Stand at 27°C for 2h.
Embodiment 2
[0032] Example 2: Dilution of recombinant baculovirus titer standard and sample to be tested
[0033] (1) Materials TC-100 medium and fetal bovine serum were purchased from GIBCO; micropipettes were purchased from EPPENDORF; EP tubes and tips were purchased from AXYGEN.
[0034] (2) Operation method
[0035] Dilute the recombinant baculovirus titer standard with TC-100 medium, the highest concentration is 320-fold dilution, and then use 2-fold gradient dilutions such as 640-fold dilution and 1280-fold dilution, a total of 8 dilutions; use TC-100 medium Dilute the sample to be tested, the highest concentration is 20-fold dilution, the following 4-fold dilutions such as 80-fold dilution, 320-fold dilution, etc., are used for a total of 5 dilutions.
Embodiment 3
[0036] Example 3: Quantitative determination of recombinant insect baculovirus titer
[0037] (1) Materials Fetal bovine serum was purchased from GIBCO; pNPP was purchased from Amersco; Triton X-100, sodium acetate, and NaOH were purchased from Sinopharm; EDTA was purchased from Shanghai Test.
[0038] (2) Operation method
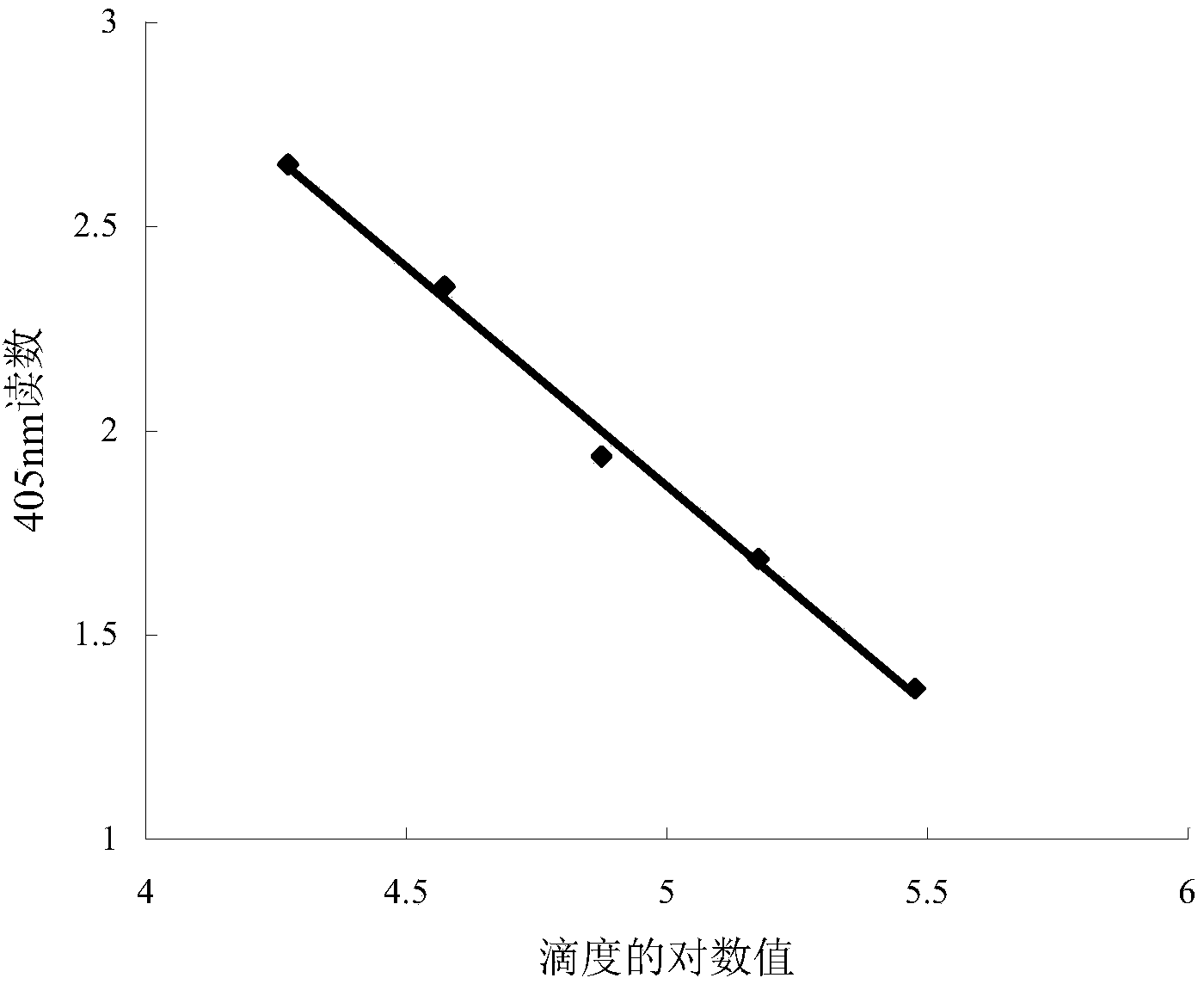
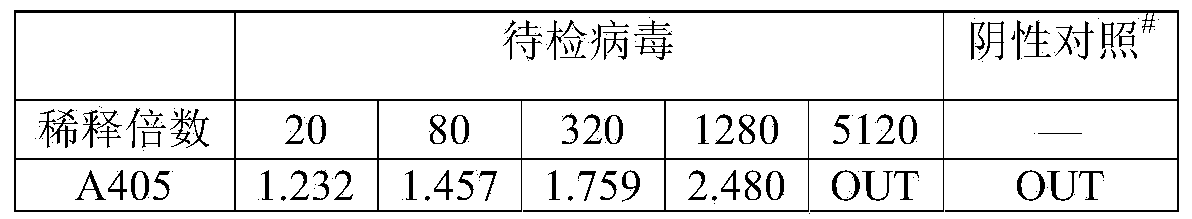
[0039] Aspirate the culture medium in the 96-well cell culture plate, add 50 μL of serially diluted virus solution to each well, and incubate at room temperature for 2 hours to allow the virus to adsorb. Add 50 μL of TC-100 medium containing 20% (V / V) fetal bovine serum to each well, and culture at 27°C for 72 hours. Aspirate the culture medium in each well, add 100 μL of pNPP solution (1mg / mL pNPP, 0.06mol / L sodium acetate, 0.001mol / LEDTA, 0.2% Triton X-100, pH6.0) in sequence, and place at 37°C for 1h. Add 10 μL of 1mol / L NaOH to each well, mix well, and place at room temperature for 5-20 minutes. Read the absorbance value at 405nm on a BIO-TEK micr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com