Acid phosphatase mutant, encoding gene, vector and application
A technology of acid phosphatase and mutant, which is applied to vectors and application fields containing the gene, can solve the problems of high cost of I+G and low enzyme activity, and achieve the effect of high practical value and broad market application prospects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
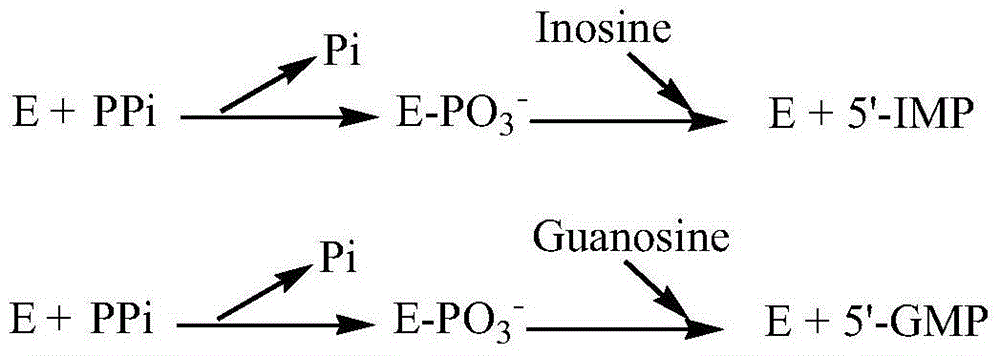
[0018] Example 1: Determination of protein crystal structure and saturation mutation point of acid phosphatase (EB-AP / PTase)
[0019] Analysis of the protein crystal structure of acid phosphatase (PDB protein database ID: 1EOI), the active sites of acid phosphatase are: Lys115, Arg122, Ser148, Gly149, His150, Arg183, His189, Asp193. Inosine was used as the substrate for molecular docking. The following three mutation points were determined: aspartic acid at position 108, glutamic acid at position 104, and asparagine at position 143.
Embodiment 2
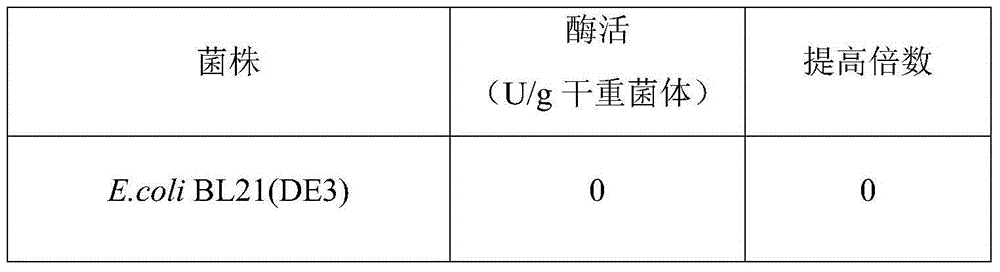
[0020] Embodiment 2: Construction of acid phosphatase mutant
[0021] (1) Construction of acid phosphatase (EB-AP / PTase) recombinant Escherichia coli
[0022] According to the sequence shown in SEQ ID No: 4, it was synthesized by a total chemical synthesis method, cloned into the expression vector pET28b, and the recombinant expression plasmid pET28b-AP / PT was transferred into E. coli E.coli BL21 (DE3). Recombinant Escherichia coli E.coli BL21(DE3) / pET28b-AP / PT was successfully constructed by enzyme digestion and colony PCR identification.
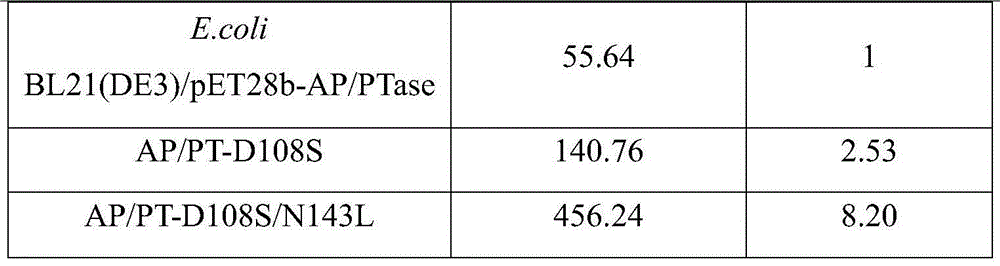
[0023] (2) Molecular modification of E.coli BL21(DE3) / pET28b-AP / PTase
[0024] A. For E.coli BL21(DE3) / pET28b-AP / PTase, amplify the target gene with primers (108):
[0025] Upstream primer: GAGGACGCCGGANNNCTTGCAACTCGT
[0026] Downstream primer: ACGAGTTGCAAGNNNTCCGGCGTCCTC
[0027] Using the plasmid of E.coli BL21(DE3) / pET28b-AP / PTase as a template and Prime STAR as a polymerase, PCR amplification was carried out with the participation...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com