Oleanane-type triadic compound and its preparation method and medical application
A kind of technology of oleanane type and compound, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] Preparation of Oleanane-Type Triadic Compounds
[0076] Dried and crushed red crest grass ( Aceriphyllum rossiiEngler) root 1.5-2.5 Kg soaked in 10L methanol at room temperature for 3 days to extract once, a total of three extractions. The combined methanol extracts were concentrated under reduced pressure to obtain extract (178g). Dissolve the extract in 1.0-0.6L water, form a suspension and extract with 3.0-2.0L n-hexane, and concentrate the n-hexane extract under reduced pressure to obtain n-hexane extract (89g); pass the n-hexane extract through 200-300 Silica gel column chromatography, using n-hexane / ethyl acetate (V / V, 10:1-0:1) as the mobile phase for gradient elution, the collected separated components were detected by silica gel thin layer chromatography, and the components were the same After combining and concentrating the separated fractions, ten separated fractions from F1 to F10 are obtained? .
Embodiment 2
[0078] Compound 1 was isolated:
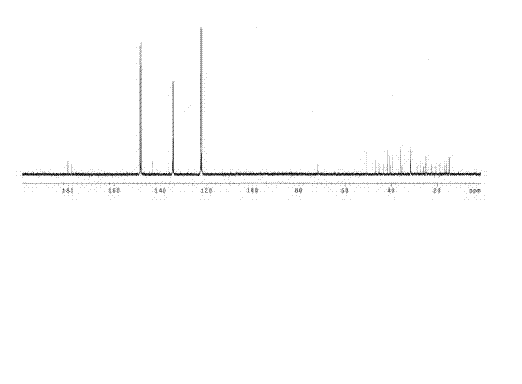
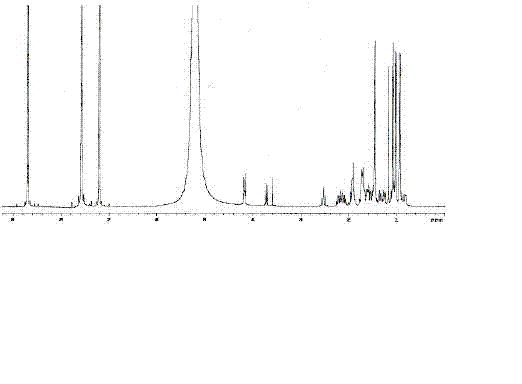
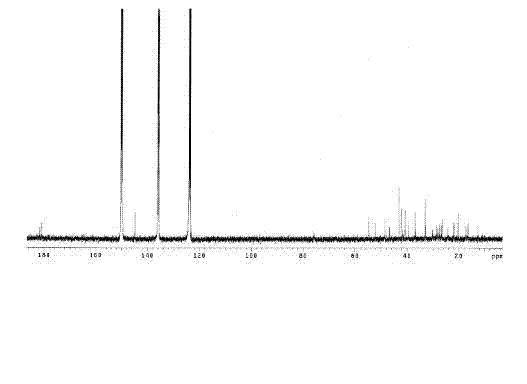
[0079] The fraction of F10 (1263.4 mg, 12-15% ethyl acetate) prepared in Example 1 was subjected to 200-300 mesh silica gel column chromatography, and chlorinated aromatic / acetone (V / V, 10:1-1:1) Gradient elution was carried out as the mobile phase, and the collected separated components were detected by silica gel thin-layer chromatography. The separated components with the same composition were combined and concentrated to obtain seven separated components from H1 to H7. The concentrated H1 component (191.2 mg, chlorophene / acetone, 10:1-10:2) was determined to be a monomeric compound, namely compound 1 (191.2 mg), by 400 MHz NMR detection of hydrogen spectrum. That 1 H and 13 C-NMR spectrum such as figure 1 , figure 2 .
Embodiment 3
[0081] Compound 2 was isolated:
[0082] Part H7 (466.9 mg, chlorophene / acetone, 4:5-5:5) prepared in Example 2 was subjected to 200-300 mesh silica gel column chromatography, and used for n-hexane / ethyl acetate (V / V, 15 :1-5:1) is the mobile phase for gradient elution, and the collected separated components are detected by silica gel thin layer chromatography. The separated components with the same composition are combined and concentrated to obtain six separated components from G1 to G6. The collected fraction of G5 (188.9 mg, n-hexane / ethyl acetate, 6:1) was subjected to reverse-phase high performance liquid chromatography using C 18 Column (10μm, 250×10 mm), with acetonitrile:H 2 O=50:1-100:1 (V / V) was used as the gradient elution of the mobile phase, the flow rate was 2mL / min, the column temperature was 25°C, and the detection was carried out at 210nm wavelength for 70min. The 22.3-26.9 min eluate was collected, and the collected solution was concentrated to obtain comp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com