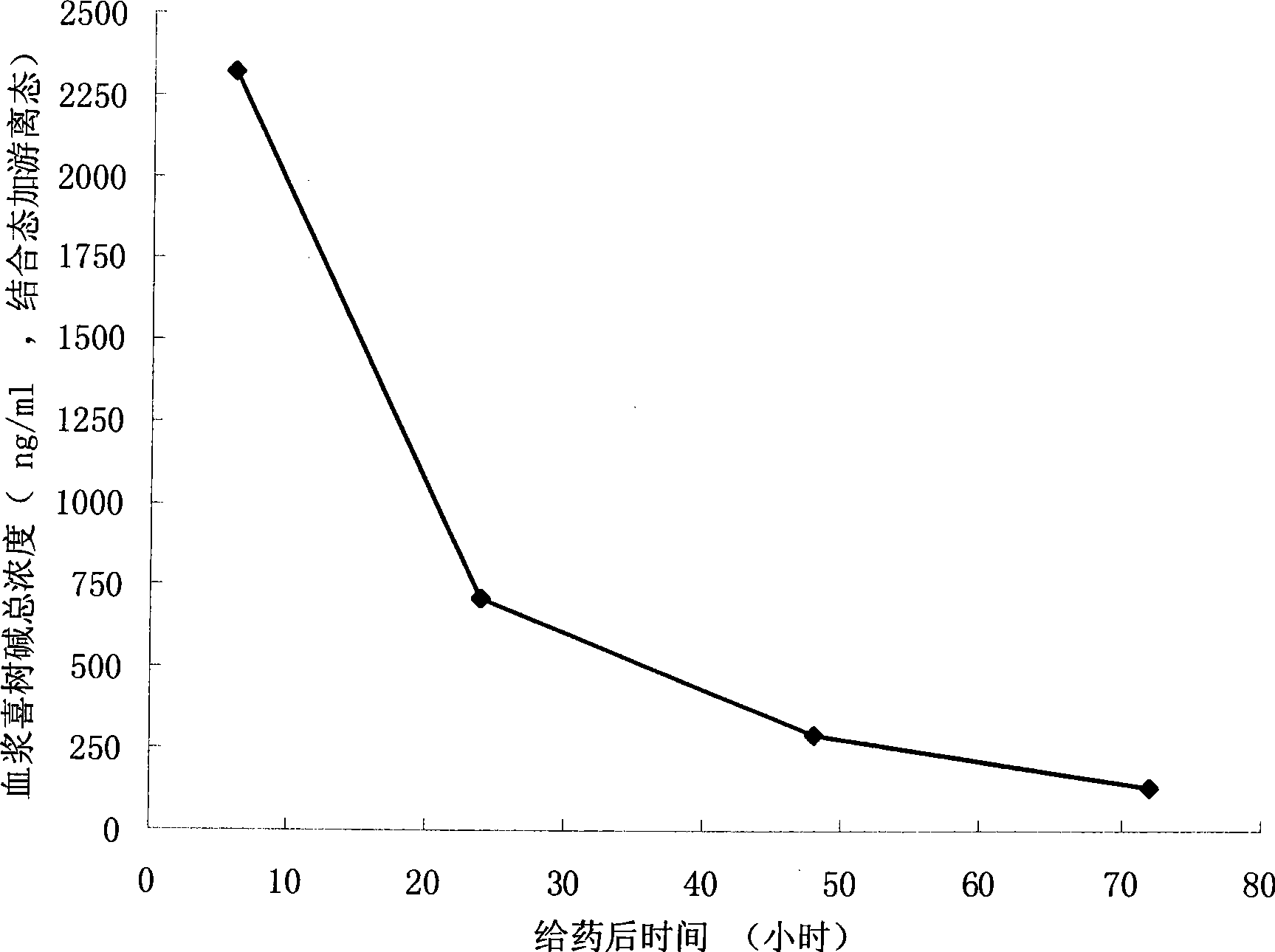
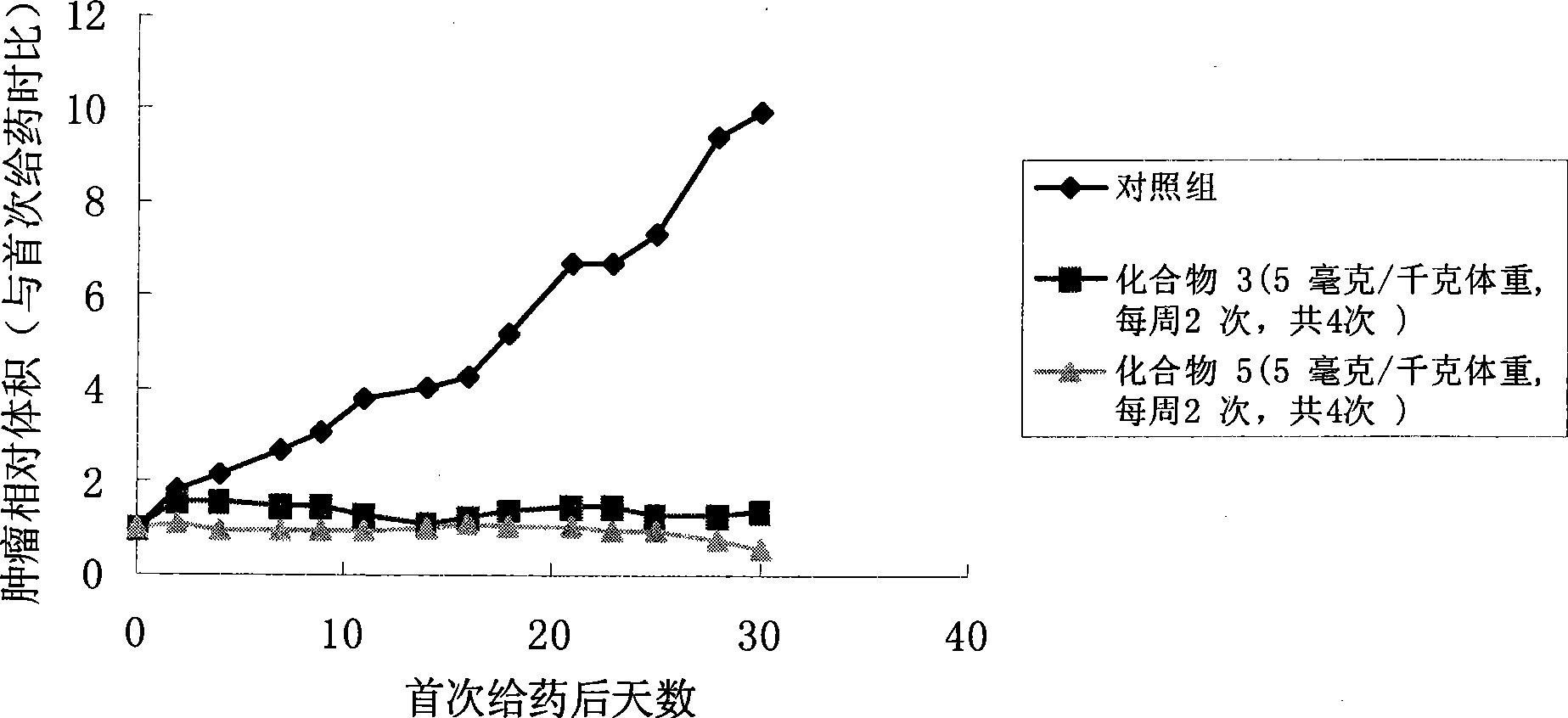
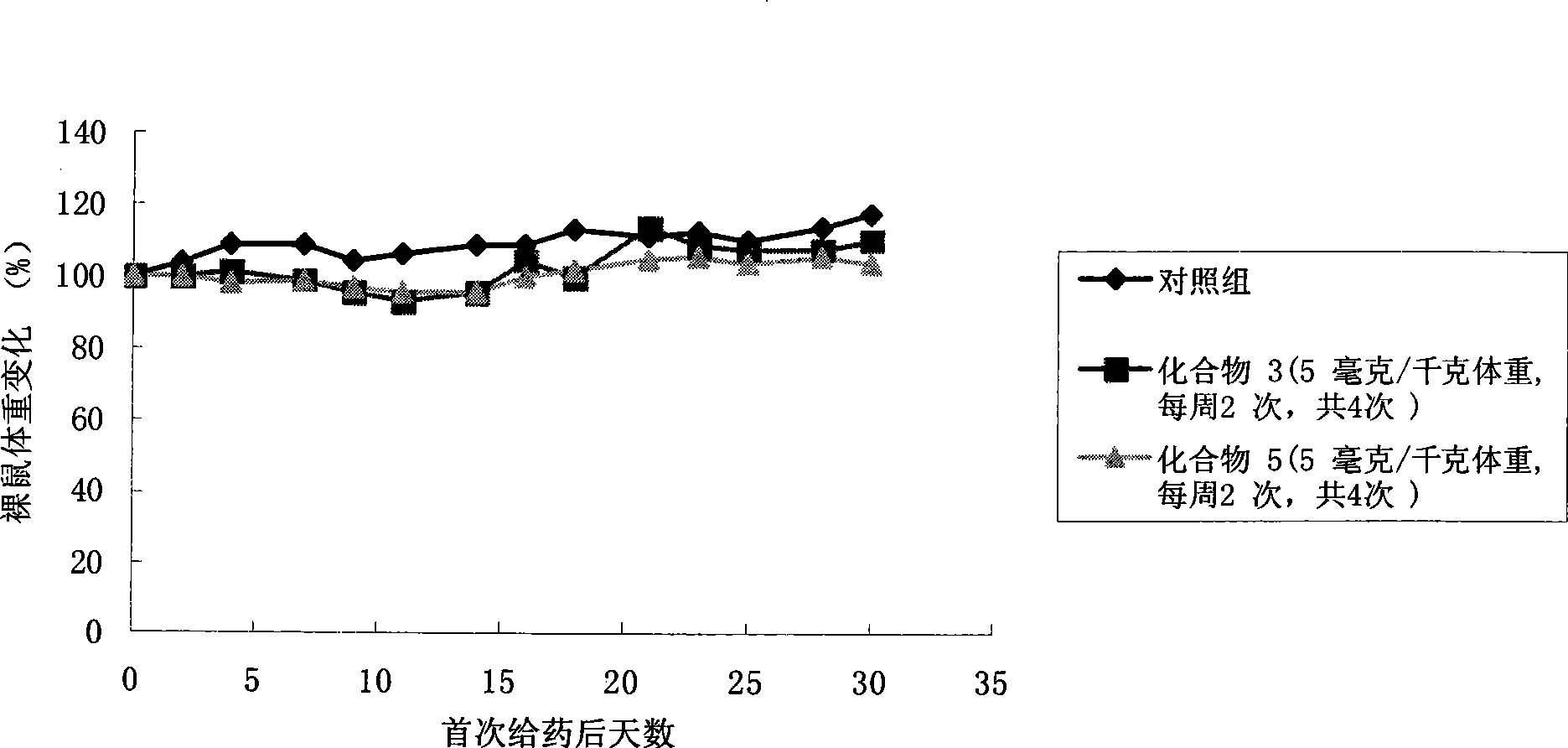
Camptothecine and non-linear polyethyleneglycol prodrug of derivative thereof
A polyethylene glycol, non-linear technology, used in anti-tumor drugs, drug combinations, pharmaceutical formulations, etc., can solve problems such as oversize, metabolic clearance and biodegradation, and large proportion of ineffective molecules
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Embodiment 1. Compound 1 (polyethylene glycol tetracarboxylic acid, 20k 4arm-PEG-OCH 2 -COOH)
[0055] Four-arm polyethylene glycol ( 20k 4arm-PEG-OH, 8.0g, 0.4mmol) was dissolved in toluene (80ml), and the toluene / water azeotrope (20ml) was distilled off under reduced pressure. The solution was cooled to 30-35°C, and potassium tert-butoxide (0.538 g, 4.8 mmol) was added. After the mixture was refluxed for 1 hour, it was cooled to 40°C, ethyl bromoacetate (1.18 g, 6.4 mmol) was added, and stirred overnight at 40°C. After the reactant was cooled to room temperature, diethyl ether was slowly added to precipitate the product. The suspension was filtered with suction, the precipitate was washed twice with ether, the solid was dissolved in 1N sodium hydroxide (50 ml), and stirred at room temperature for 2 hours to obtain a hydrolyzate. After the hydrolyzate was acidified with hydrochloric acid, it was extracted three times with dichloromethane (DCM) (25mlX3). The combi...
Embodiment 2
[0056] Embodiment 2. Compound 2 (polyethylene glycol octacarboxylic acid, 20k 8arm-PEG-OCH 2 -COOH)
[0057] The preparation process of Compound 2 is basically the same as that of Compound 1 in Example 1 above, except that the molecular ratio of potassium tert-butoxide and ethyl bromoacetate to polyethylene glycol is doubled.
Embodiment 3
[0058] Embodiment 3. Compound 3 (20- 20k 4arm-PEG-OCH 2 -C(O)CPT)
[0059] Compound 1 (500mg, 0.025mmol) was dissolved in toluene (15ml), evaporated under reduced pressure to remove the toluene / water azeotrope (10ml), cooled, added dichloromethane (15ml), camptothecin (CPT, 52.2mg, 0.15mmol ). The mixture was cooled to 0°C, N-ethyl-N'-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDC, 28.7mg, 0.15mmol) and dimethylaminopyridine (DMAP, 18.3 mg, 0.15 mmol), the ice-water bath was removed, the reaction mixture was gradually warmed to room temperature, and stirring was continued overnight. The reaction solution was washed with dilute hydrochloric acid, dried over anhydrous magnesium sulfate, the solvent was evaporated under reduced pressure, the residue was dissolved in methanol, and subjected to silica gel column chromatography. The eluates were combined and concentrated by evaporation. The concentrated solution was precipitated with diethyl ether, the precipitate was s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com