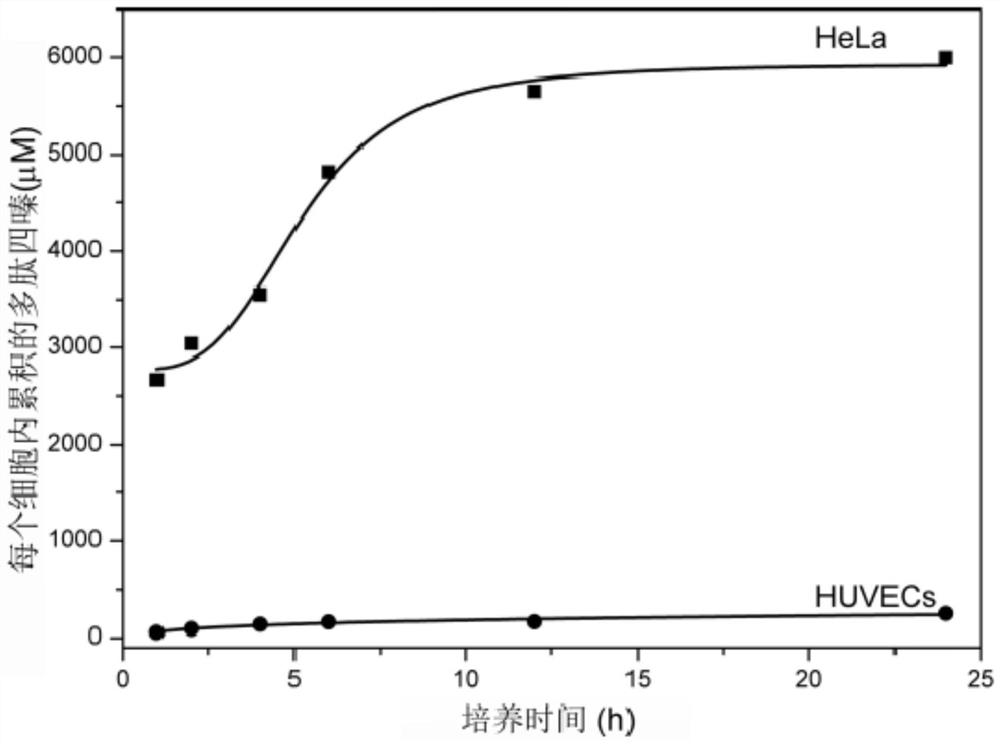
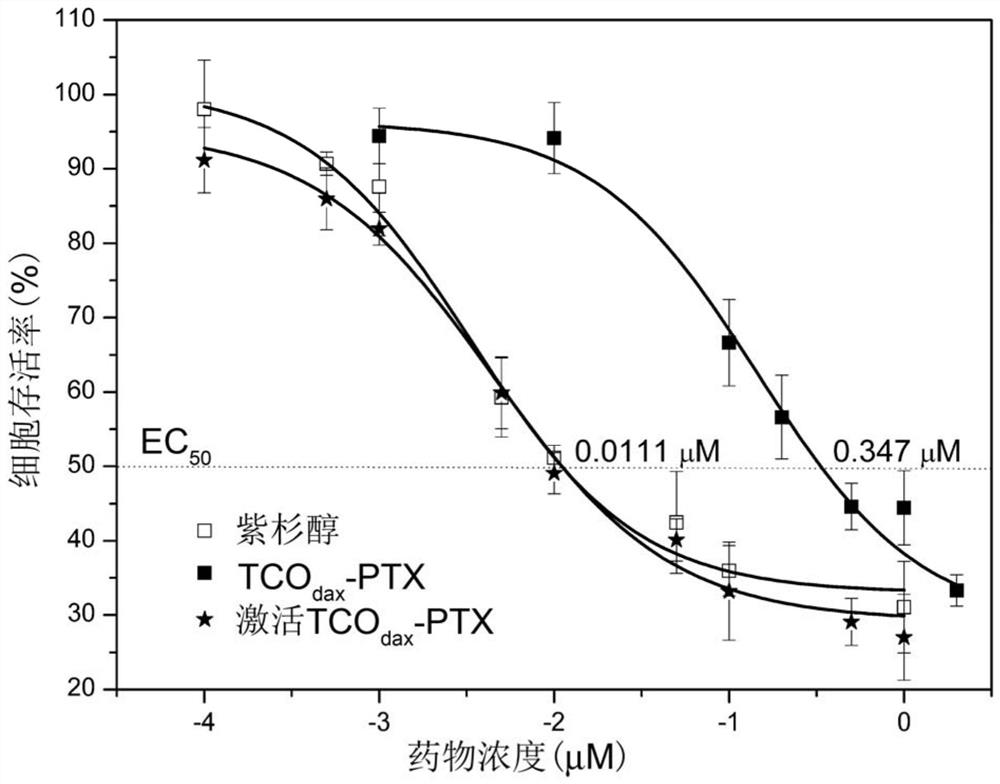
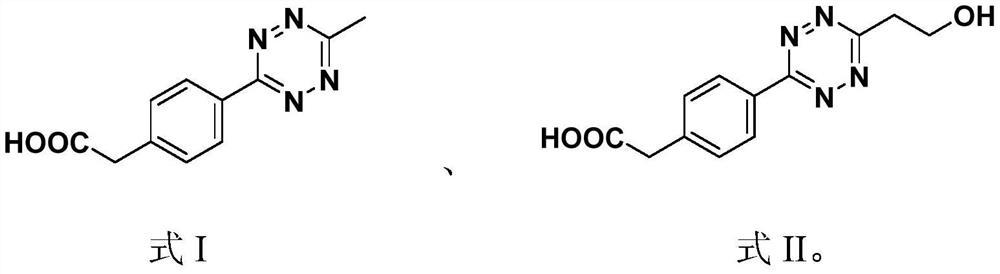
A kind of prodrug activation compound, prodrug system and its preparation method and application
A compound and aromatic compound technology, applied in the field of chemical medicine, can solve the problems of toxic side effects, low efficiency of antibody loading drug, complicated operation, etc., and achieve the effect of good biocompatibility, no systemic toxicity, and high chemical purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0081] In this example, a prodrug-activating compound with the structure shown in formula III was prepared,
[0082]
[0083] The polypeptide fragment is formed by the condensation of phosphorylated tyrosine, lysine and phenylalanine, the hydrophobic group is obtained by forming an amide bond between 2-naphthylacetic acid and the amino group in the polypeptide fragment, and the tetrazine group is formed by 2-[4 -(6-Methyl-1,2,4,5-tetrazin-3-yl)]phenylacetic acid and the amino group in the polypeptide fragment form an amide bond to obtain it.
[0084] Concrete preparation method comprises the following steps:
[0085] (1) In a solid-phase synthesis tube, swell 2-chloro-trityl chloride resin in dichloromethane for 30 min; use phosphorylated tyrosine, phenylalanine, and terminal amino groups protected by Fmoc for terminal amino groups The lysine protected by Fmoc and Boc is used as the raw material for the amino group and the side chain amino group respectively. According to ...
Embodiment 2
[0094] In this example, a prodrug-activating compound with a structure as shown in formula IV was prepared,
[0095]
[0096] The polypeptide fragment is formed by the condensation of phosphorylated tyrosine, lysine and phenylalanine, the hydrophobic group is obtained by forming an amide bond between 2-naphthylacetic acid and the amino group in the polypeptide fragment, and the tetrazine group is formed by 2-[4 -(6-ethanol-1,2,4,5-tetrazin-3-yl)]phenylacetic acid and the amino group in the polypeptide fragment to form an amide bond.
[0097] Concrete preparation method comprises the following steps:
[0098] (1) In a solid-phase synthesis tube, swell 2-chloro-trityl chloride resin in dichloromethane for 30 min; use phosphorylated tyrosine, phenylalanine, and terminal amino groups protected by Fmoc for terminal amino groups The lysine protected by Fmoc and Boc is used as the raw material for the side chain amino group and the side chain amino group respectively. According t...
Embodiment 3
[0104] In this example, a prodrug-activating compound with the structure shown in formula V was prepared,
[0105]
[0106] Wherein the polypeptide fragment is formed by the condensation of phosphorylated tyrosine, lysine and phenylalanine, the hydrophobic group is obtained by forming an amide bond from pyreneacetic acid and the amino group in the polypeptide fragment, and the tetrazine group is formed by 2-[4-( 6-methyl-1,2,4,5-tetrazin-3-yl)]phenylacetic acid and the amino group in the polypeptide fragment to form an amide bond.
[0107] Concrete preparation method comprises the following steps:
[0108] (1) In a solid-phase synthesis tube, swell 2-chloro-trityl chloride resin in dichloromethane for 30 min; use phenylalanine, phosphorylated tyrosine, and terminal amino group protected by Fmoc and side chain amino groups are respectively made of Fmoc and Boc-protected lysine as raw materials. According to the amino acid sequence of phenylalanine-phosphorylated tyrosine-ly...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com