Compositions for inhibiting masp-2 dependent complement acitivation
A technology of MASP-2 and complement activation, which is applied in the direction of introducing foreign genetic material using carriers, drug combinations, and medical preparations containing active ingredients, etc. It can solve molecular determinants that are difficult to determine and share due to the complexity and diversity of carbohydrate structures And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0378] This example describes the recombinant expression and protein production of recombinant full-length human, rat, and mouse MASP-2, MASP-2-derived polypeptides, and catalytically inactive mutant forms of MASP-2.
[0379] Expression of full-length human and rat MASP-2:
[0380] The human MASP-2 full-length cDNA sequence (SEQ ID NO: 1) with the leader sequence (SEQ ID NO: 2) encoding the human MASP-2 polypeptide was subcloned into the mammalian expression vector pCI-Neo (Promega), which Eukaryotic expression is driven under the control of the CMV enhancer / promoter region (described in Kaufman R.J. et al., Nucleic Acids Research 19:4485-90, 1991; Kaufman, Methods in Enzymology, 185:537-66 (1991)). The rat MASP-2 full-length cDNA (SEQ ID NO: 4) encoding the rat MASP-2 polypeptide with a leader sequence (SEQ ID NO: 5) was subcloned into the pED expression vector. The MASP-2 expression vector was then transfected into the adherent Chinese hamster ovary cell line DXB1 using the...
Embodiment 2
[0393] This example describes the screening method used to identify high affinity fully human anti-MASP-2 scFv antibody candidates that block MASP-2 functional activity to progress to affinity maturation.
[0394] Background and rationale:
[0395] MASP-2 is a complex protein with many individual functional domains, including: one or more MBL and ficolin binding sites, serine protease catalytic site, proteolytic substrate C2 binding site, proteolytic substrate C4 binding site, MASP-2 cleavage site for MASP-2 zymogen self-activation and two Ca ++ binding site. scFv antibody fragments were identified as binding MASP-2 with high affinity, and the identified Fab2 fragments were tested in functional assays to determine whether they were able to block MASP-2 functional activity.
[0396] In order to block MASP-2 functional activity, an antibody or scFv or Fab2 antibody fragment must bind to and interfere with a structural epitope on MASP-2 that is required for MASP-2 functional ac...
Embodiment 3
[0453] This example describes the MASP-2 functional screening method used to analyze the ability of high affinity fully human anti-MASP-2 scFv antibody candidates to block MASP-2 activity in normal human serum.
[0454] Rationale / Background
[0455] Assay to Measure Inhibition of Lectin Pathway C3 Convertase Formation
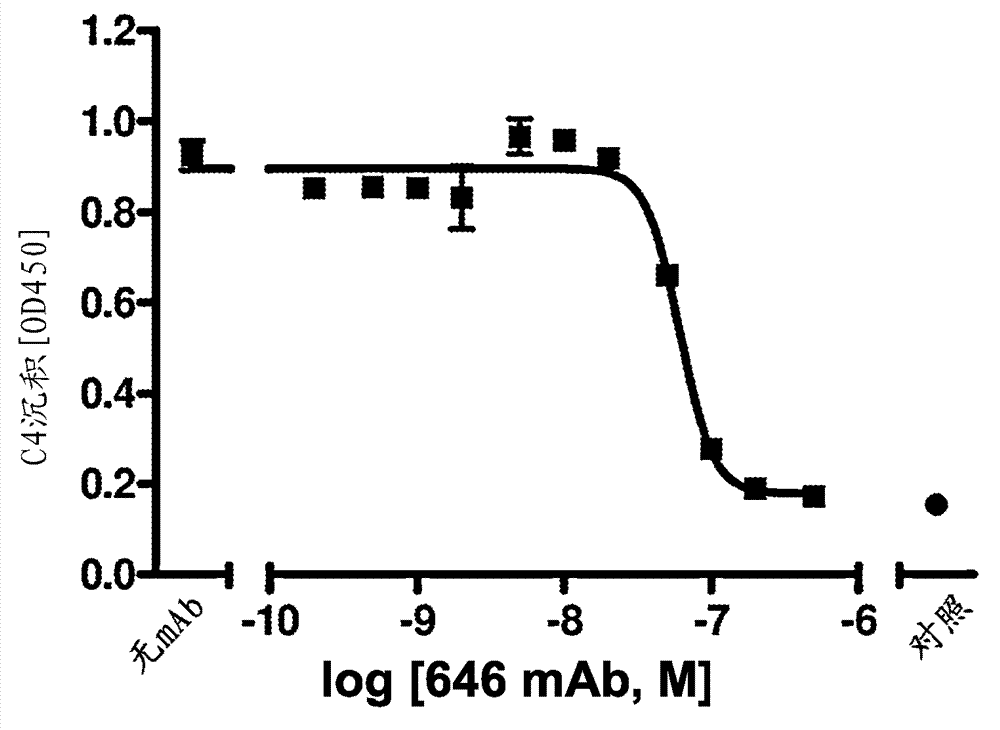
[0456]A functional assay measuring inhibition of lectin pathway C3 convertase formation was used to evaluate the "blocking activity" of anti-MASP-2 scFv candidate clones. The lectin pathway C3 convertase is an enzyme complex (C4b2a) that proteolytically cleaves C3 into two potent pro-inflammatory fragments, anaphylatoxin C3a and opsonic C3b. The formation of C3 convertase appears to be a critical step in the lectin pathway in mediating inflammation. MASP-2 is not a structural component of the lectin pathway C3 convertase (C4b2a); therefore an anti-MASP-2 antibody (or Fab2) will not directly inhibit the activity of an already existing C3 convertase. However...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com