CAR-T cell targeting ErbB receptor family and self-expressing PD-1 antibody and use thereof
A cell and antibody technology, applied in the fields of genetic engineering and immunology, can solve the problems of low cost, high production cost and response rate.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
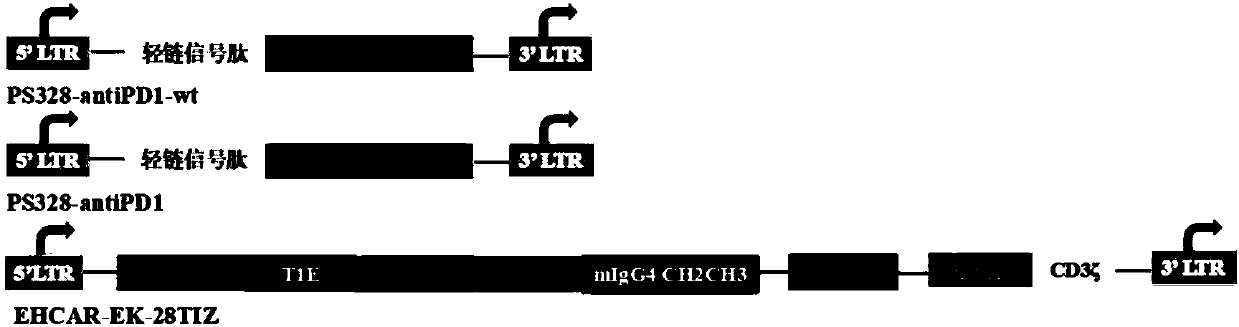
[0111] Example 1: Construction of recombinant plasmids pS328-antiPD1, pS328-antiPD1-wt and pNB328-EHCAR-EK-28TIZ
[0112] Commissioned Shanghai Jierui Biological Co., Ltd. to synthesize EHCAR-EK-28TIZ gene, anti-PD1 gene and anti-PD1-wt gene, the structural model is as follows figure 1 shown. Each gene was loaded into the pNB328 and pS328 vectors which were double digested with EcoR1+SalI (see CN 201510638974.7 for the structure and sequence of pNB328, which is incorporated herein by reference in its entirety; compared with pNB328, pS328 lacks PB transposition subsequence, and other elements are the same as the pNB328 vector), construct plasmids, respectively named as pNB328-EHCAR-EK-28TIZ, pS328-antiPD1 and pS328-antiPD1-wt.
[0113] The nucleotide sequence of the light chain signal peptide in the structural model diagram is shown in the 1-60 base sequence of SEQ ID NO:2; the encoding of Anti-PD1-wt is shown in SEQ ID NO:4 (the amino acid sequence is shown in SEQ ID NO: 3 s...
Embodiment 2
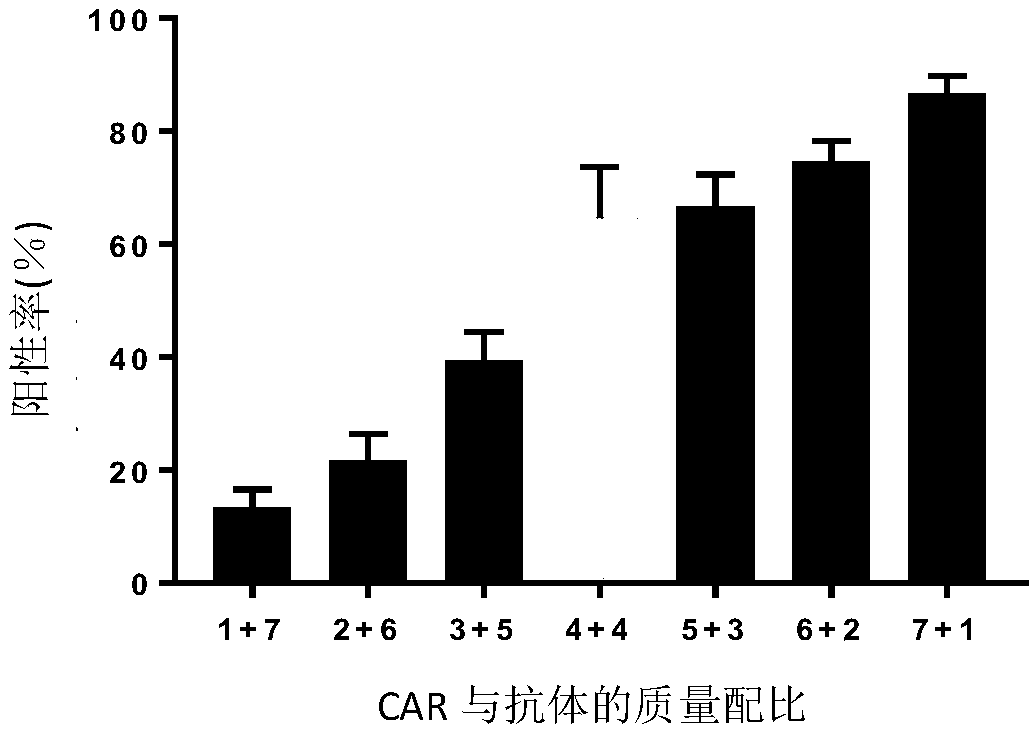
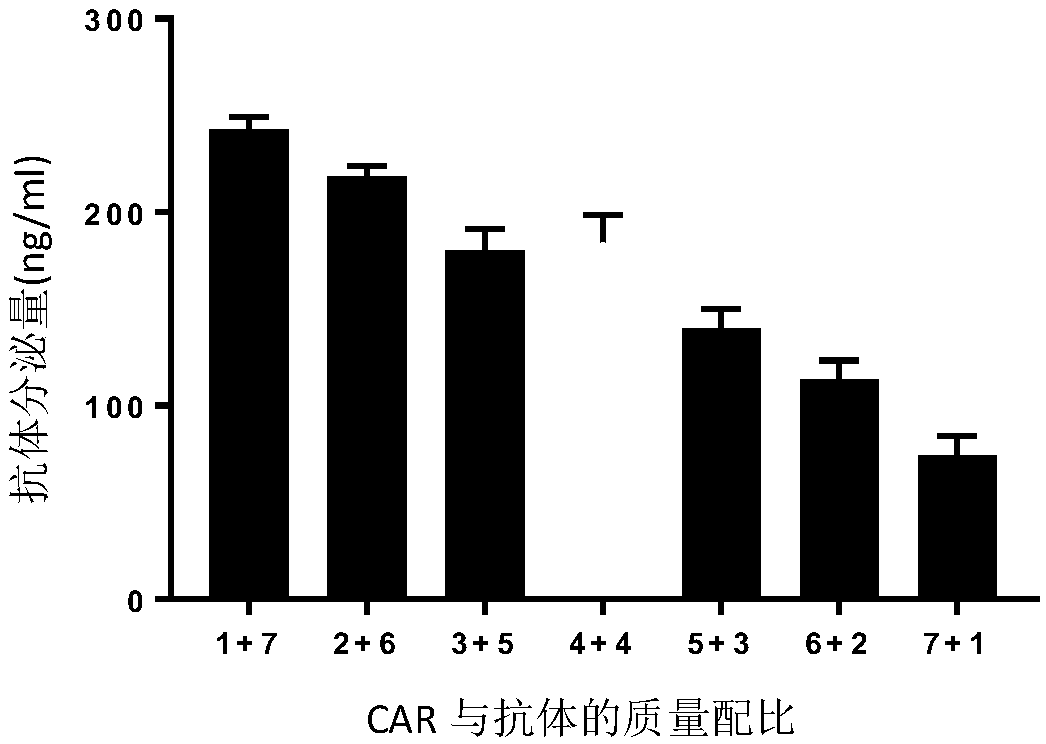
[0114] Example 2: Determination of positive rate and antibody expression of chimeric antigen receptor-modified T cells constructed by pNB328-EHCAR-EK-28TIZ and pS328-antiPD1 plasmids with different mass ratios
[0115] Set the amount of pNB328-EHCAR-EK-28TIZ and pS328-antiPD1 plasmids to 7 ratios of 1ug+7ug, 2ug+6ug, 3ug+5ug, 4ug+4ug, 5ug+3ug, 6ug+2ug, 7ug+1ug , Carry out CART cell construction. The build method is as follows:
[0116] Peripheral blood mononuclear cells (PBMCs) were isolated from Shanghai Cell Therapy Production Center. Cultivate PBMCs for 2-4 hours. The unattached suspension cells are initial T cells. Collect the suspension cells into a 15ml centrifuge tube, centrifuge at 1200rmp for 3min, discard the supernatant, add physiological saline, centrifuge at 1200rmp for 3min, discard the physiological saline, and repeat this step; take eight 1.5ml centrifuge tubes and add 5×10 6 Cells, numbered a, b, c, d, e, f, g and h, centrifuged at 1200rmp for 3min, discard...
Embodiment 3
[0134] Example 3: Construction of EHCAR-EK-28TIZ T cells and EHCAR-EK-28TIZ-antiPD1T cells and determination of positive rate and antibody expression
[0135]Take 6ug pNB328-EHCAR-EK-28TIZ plasmid for the construction of EHCAR-EK-28TIZ T cells, respectively take 4ugpNB328-EHCAR-EK-28TIZ and 4ug pS328-antiPD1 plasmids for the construction of EHCAR-EK-28TIZ-antiPD1T cells, construction method With embodiment 2.
[0136] The positive rate of EHCAR-EK-28TIZ T cells and EHCAR-EK-28TIZ-antiPD1T cells was detected by flow cytometry, and the method was the same as in Example 2. see results Figure 3A , Self-expression of PD1 antibody does not reduce the positive rate of CART cells.
[0137] ELISA detection EHCAR-EK-28TIZ-antiPD1 T cell antibody expression, the method is the same as in Example 2, see the results Figure 3B .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com