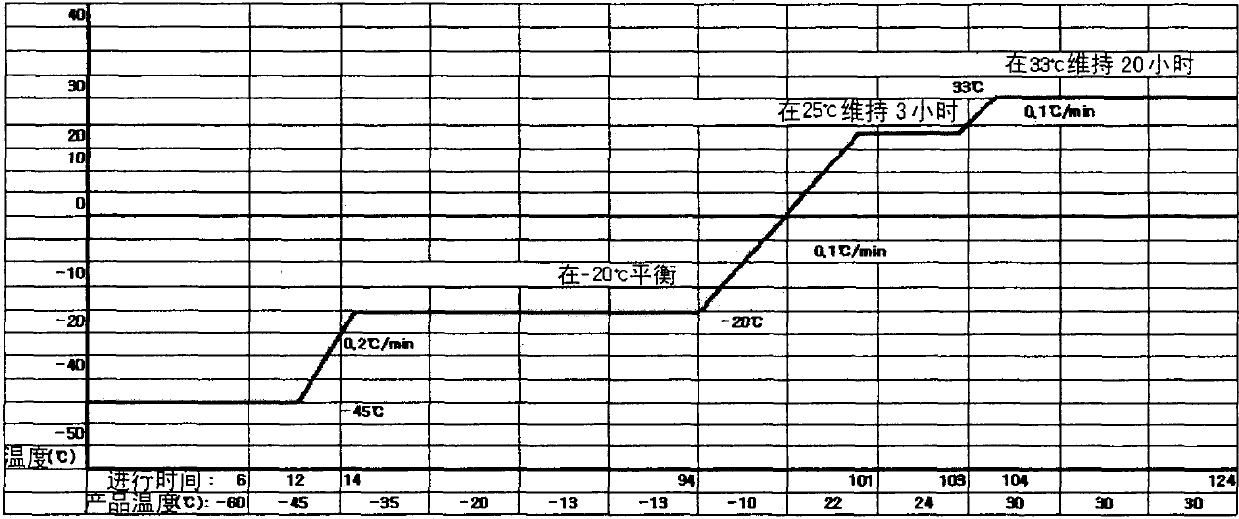
Pharmaceutical preparation of recombinant factor VIII lyophilized without albumin as a stabilizer
A coagulation factor, drying technology, applied in the direction of coagulation/fibrinolysis factor, freeze-dried delivery, factor VII, etc., can solve problems such as virus infection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] In order to search for a substance that can stabilize recombinant human coagulation factor VIII instead of albumin derived from human blood, lecithin (phosphatidylcholine), sucrose, glycine, PVP (polyvinylpyrrolidone) and amino acid (arginine) were used as stabilizers. amino acid, isoleucine, glutamic acid) to prepare the final stock solution mixture for freeze-drying of each recombinant human coagulation factor VIII, and in order to confirm the activity after freeze-drying, for each prepared recombinant human coagulation factor VIII after freeze-drying The human factor VIII coagulation mixture was tested for activity, cake apperance, and clarity.
[0045] The materials and instruments used are as follows.
[0046] -L-Arginine (M.W.210.7 Sigma)
[0047] -L-Isoleucine (M.W.131.2 Sigma)
[0048] -L-Glutamic acid (M.W.169.1 Sigma)
[0049] -L-Histidine hydrochloride (M.W.209.63 Fluka)
[0050] - Sucrose (M.W. 342.3 Sigma)
[0051] -Tween 80 (Sigma)
[0052] -PEG 3350...
Embodiment 2
[0087] In order to search for the amino acid (arginine, isoleucine, glutamic acid) recombinant human coagulation factor VIII mixture that exhibits optimal conditions as a substitute for the albumin stabilizer as shown in the above-mentioned Example 1 Experiments were conducted on the best conditions for the blocky appearance after freeze-drying, and the experimental results are shown in Table 3 below.
[0088] Exterior
[0089] As shown in Table 3 above, compared with the control sample (containing albumin), the condition of arginine 12-36mM, isoleucine 4-18mM, and glutamic acid 19-57mM showed the best lumpy appearance .
Embodiment 3
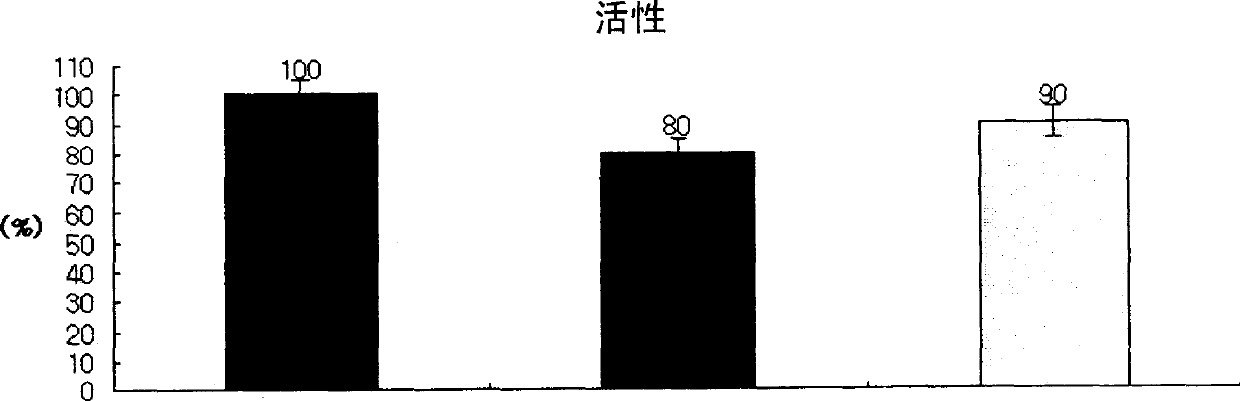
[0091] With reference to the results in Table 3 above, among the three amino acids, the concentration of two amino acids was kept constant at the minimum concentration (arginine 12 mM, isoleucine 3.5 mM, glutamic acid 19 mM) to form a block appearance, and in order to Amino acid, isoleucine, glutamic acid each concentration investigation activity change, change the concentration of a kind of amino acid, carry out freeze-drying as above Example 1 and make the recombinant human coagulation factor VIII mixture, and according to chromatographic analysis and Coagulation Assays The activity expressed as % relative to the recombinant human factor VIII coagulation mixture containing albumin was analyzed.
[0092] The concentration of isoleucine and glutamic acid were kept constant at the minimum concentration of 3.5mM and 19mM, which formed a complete block appearance after freeze-drying, and only the concentration of arginine was changed in the range of 6-100mM to evaluate its activit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com