Compositions and methods for the treatment of hemophilia a
a technology of hemophilia and composition, applied in the field of medicine and gene therapy, can solve problems such as compromised vital organs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example i
Novel Minigene Encoding Improved Factor VIII
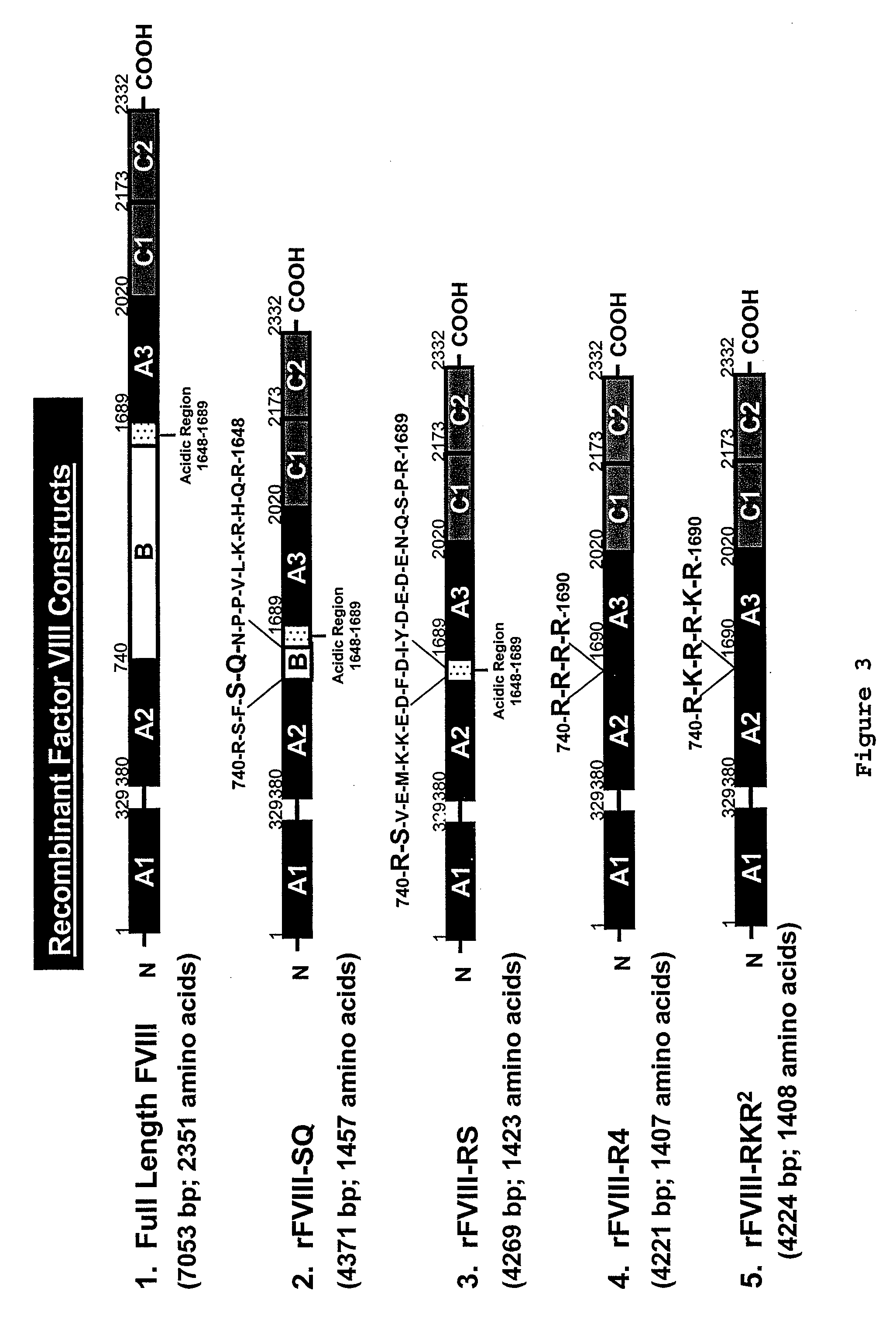
[0084] In accordance with the present invention, new recombinant Factor VIII minigenes are provided which stimulate production of Factor VIII in cells comprising the minigene with higher activity than prior art recombinant constructs.
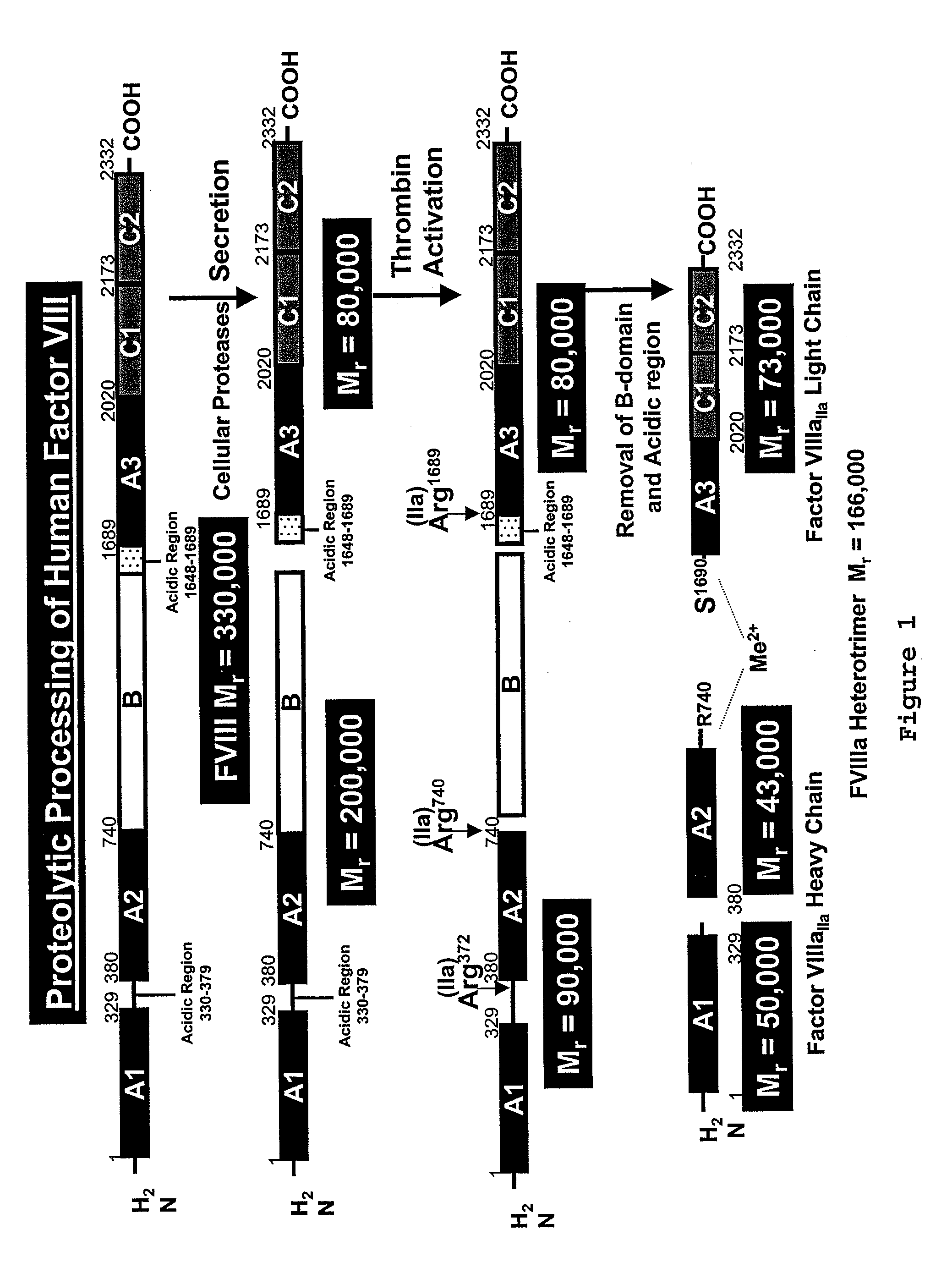
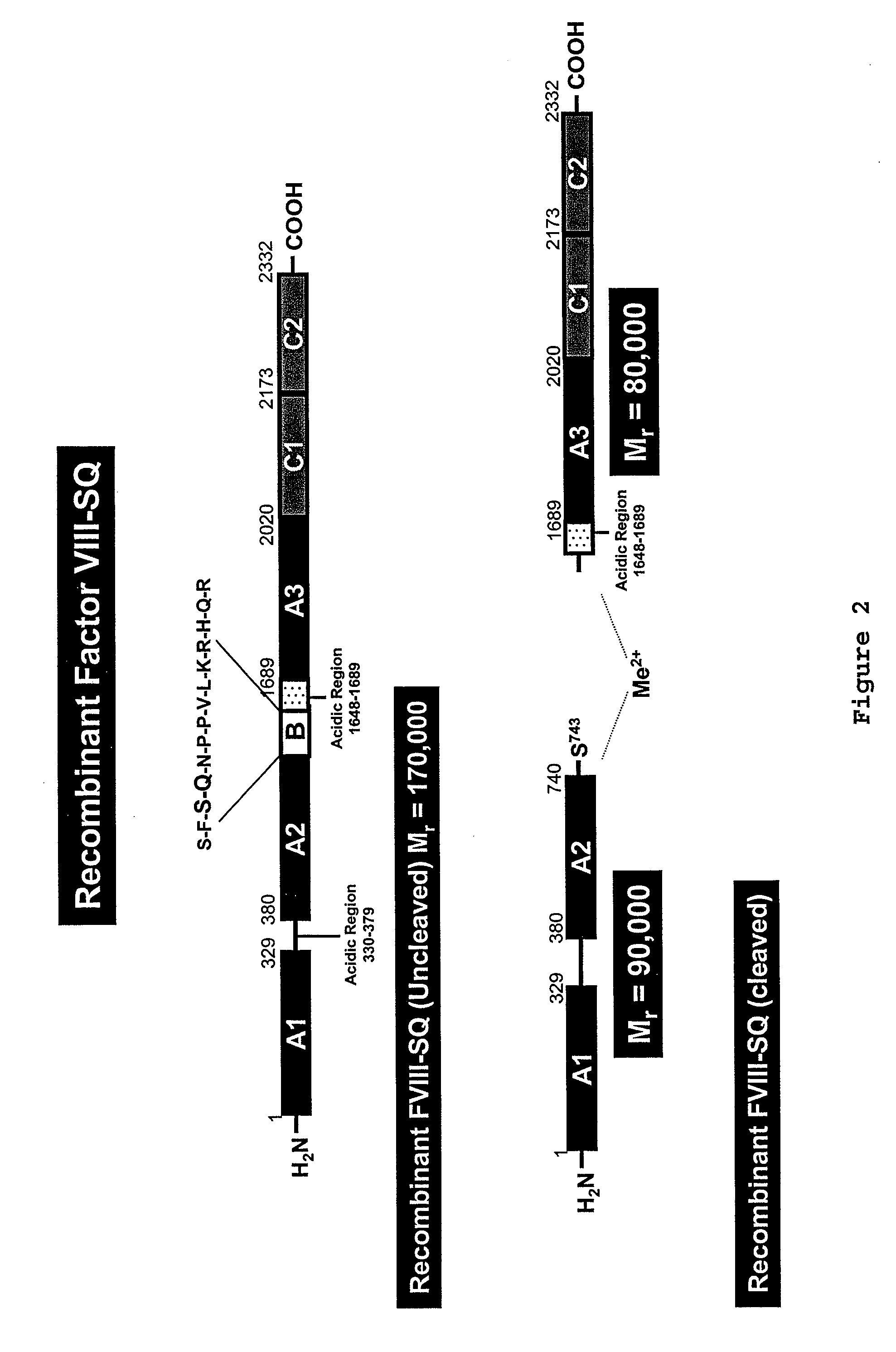
[0085] Native FVIII is synthesized as a single chain polypeptide (2332 amino acids) preceded by a 19-residue signal sequence and has a molecular weight of Mr=330,000. The signal sequence is removed upon translocation of FVIII into the ER and the native FVIII is then cleaved in the B-domain in connection with its secretion. This results in the release of a heterodimer comprised of a Mr=200,000 heavy chain and a Mr=80,000 light chain, the association of which is metal-ion dependent. See FIG. 1.
Creation of the FVIII Minigenes:
[0086] The FVIII cDNA in the expression plasmid pSP64 was purchased from the ATCC. Following a Sal I restriction digest, the FVIII cDNA was subcloned into the mammalian expression plasm...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular mass | aaaaa | aaaaa |
| molecular mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com