Kit for measuring the thrombin generation in a sample of a patient's blood or plasma
a thrombin generation and kit technology, applied in the field of kits for measuring can solve the problems of no direct monitoring of the drug substance for either treatment regime, no response, and patients with haemophilia a who develop inhibitors, and achieve the same sensitivity in the generation of thrombin, simple and efficient assay, fast and reproducible
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
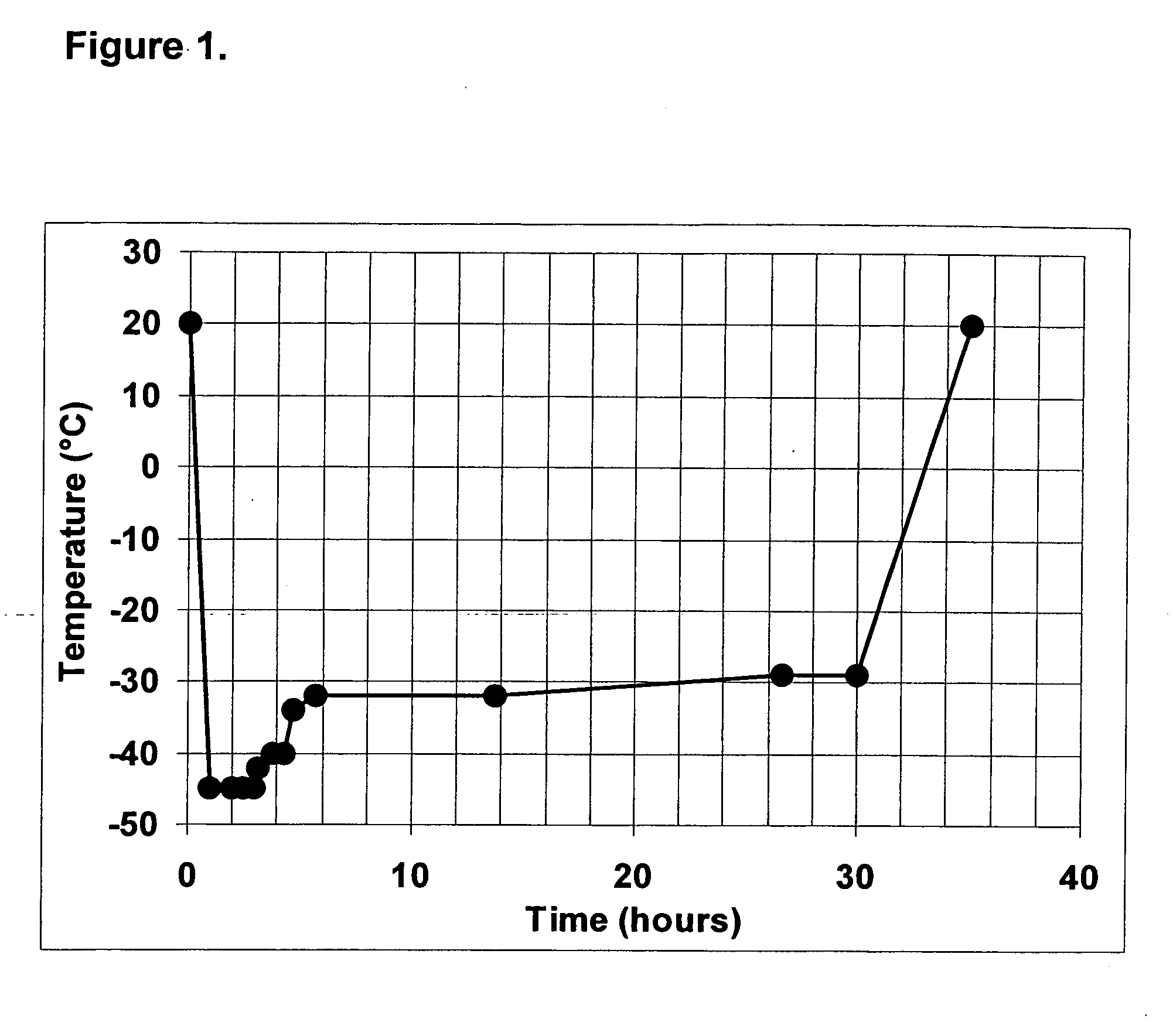
Preparation of a Frozen and a Lyophilized TF / PL-Complex
[0048] A tissue factor having phospholipid vesicles (TF / PL-complex) is prepared by using a recombinant full-length TF (American Diagnostica Inc. Greenwich, Conn., U.S.A.) and synthetic PLs (Avanti Polar Lipids, Alabaster, Ala., U.S.A.). The preparation comprises the following steps:
[0049] Phospholipid vesicles composed of 1,2-Dioleyl-sn-glycero-3-phosphocholine (DOPC), 1-Palmitoyl-2-oleyl-sn-glycero-3-phosphoserine (POPS) and 1,2-Dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE) (Avanti Polar Lipids, Alabaster, Ala.) are prepared by the extrusion method of Hope et al. (Hope M J, Bally M B, Webb G, Cullis P R: “Production of large unilamellar vesicles by a rapid extrusion procedure. Characterization of size distribution, trapped volume and ability to maintain a membrane potential.” Biochim Biophys Acta 812:55, 1985) using an extrusion device of Lipex Biomembranes, Inc. (Vancouver, Canada) equipped with two stacked polycarbonate ...
example 2
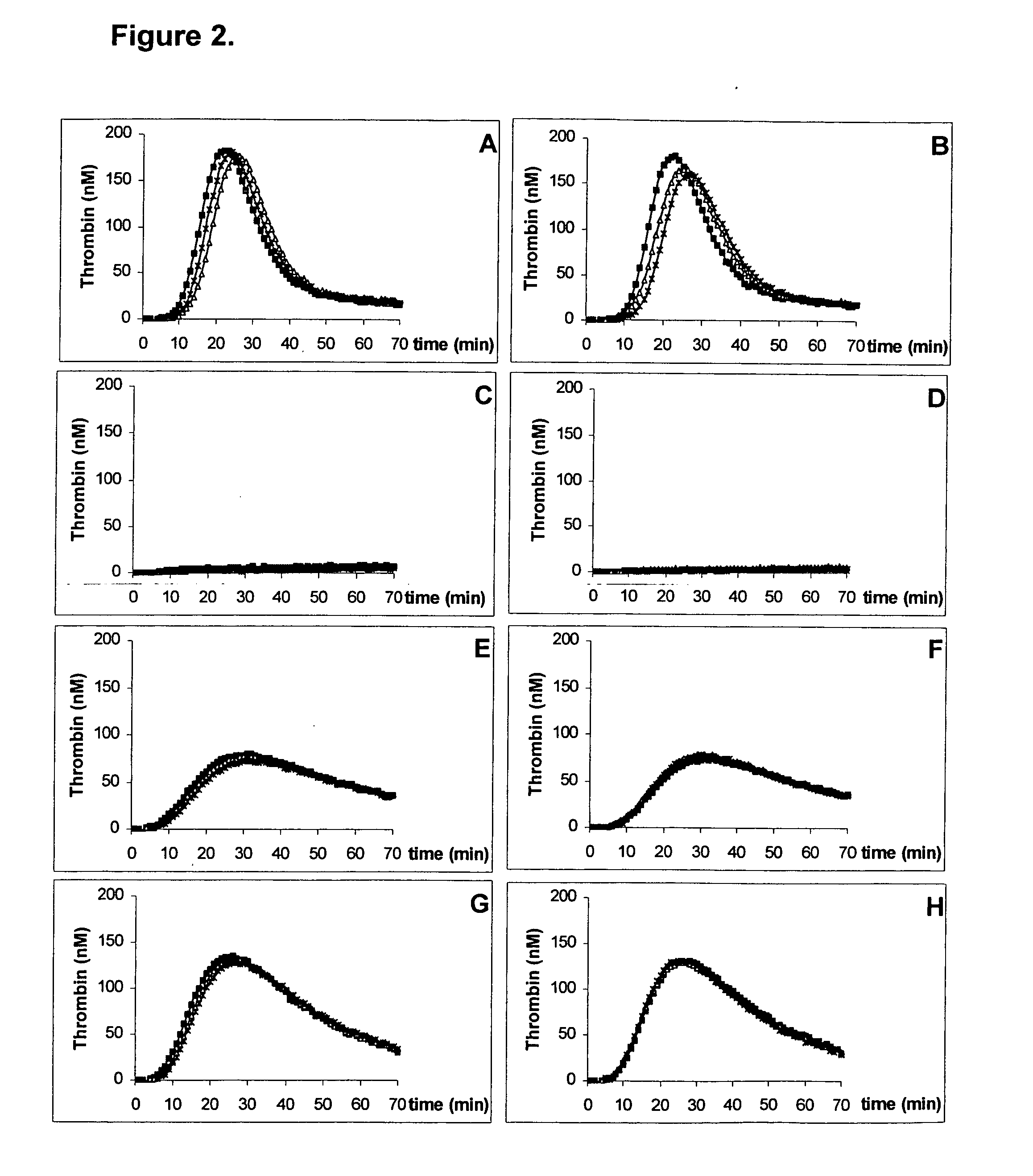
Thrombin Generation Triggered with Frozen or Lyophilized TF / PL-Complex in Various Plasma Samples
[0052] The thrombin generation is triggered by a TF / PL-complex, prepared as described above containing 18 pM TF and 3.2 μM PL, wherein the PL is composed of a ratio of 80% by weight DOPC and 20% by weight POPS. The lyophilized TF / PL-complex is dissolved in water for injection (to a final concentration of 18 pM TF and 3.2 μM PL) and 10 μL of this aqueous solution is added to 50 μL of 1 mM thrombin substrate Z-Gly-Gly-Arg-AMC (Bachem AG, Bubendorf, Switzerland) premixed with 15 mM CaCl2. For comparison 10 μL of a frozen TF / PL-complex (18 pM TF and 3.2 μM PL) is mixed with 50 μL of the above mentioned thrombin-substrate. The addition of 40 μL plasma sample starts the reaction. The components are incubated at 37° C.
[0053] The thrombin-substrate is cleaved by the generated thrombin and a fluorophore-containing moiety is released. The increase of the fluorescence intensity, which is proportio...
example 3
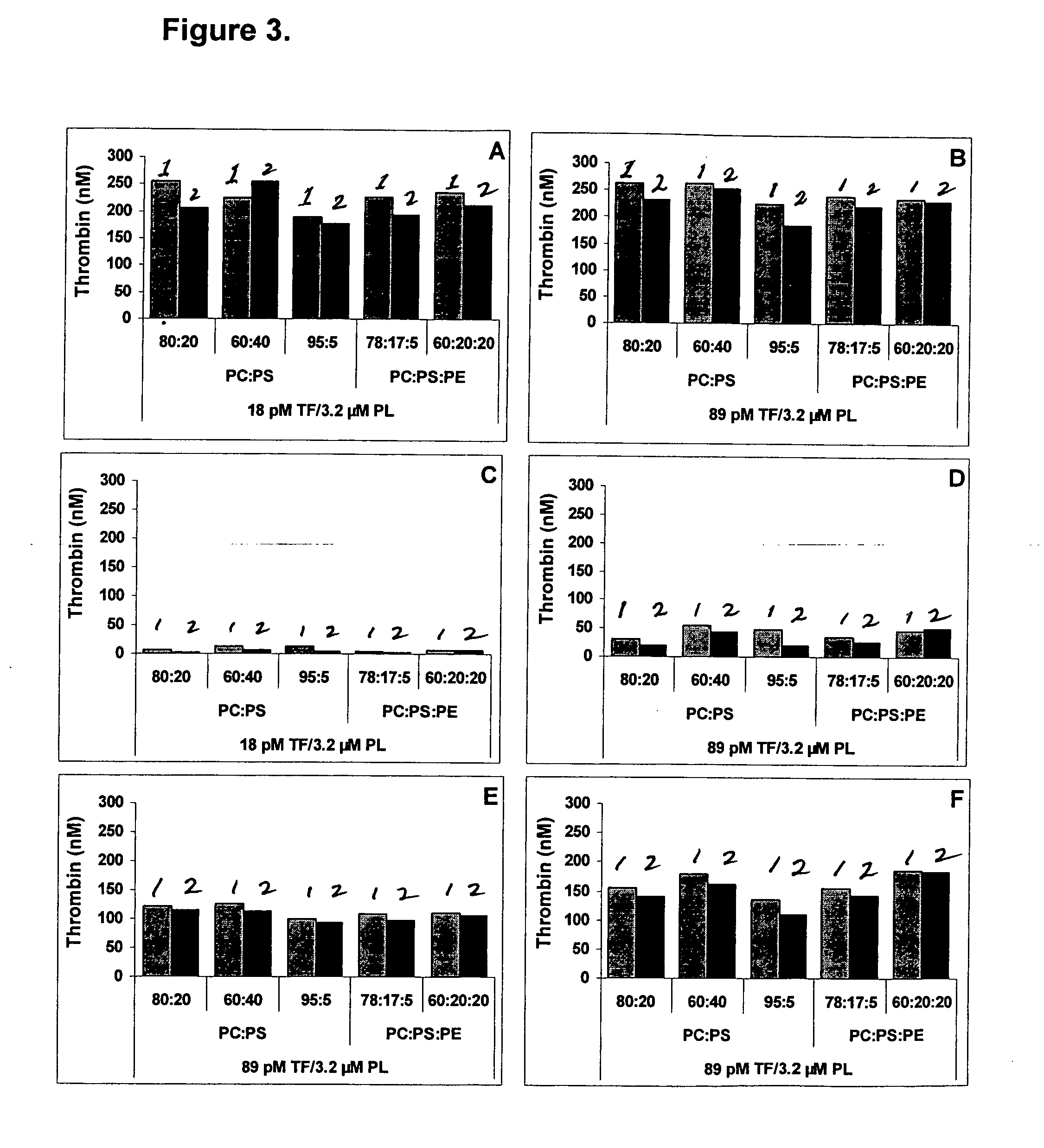
Comparison of the Thrombin Generation Triggering Effect of Frozen and Lyophilized TF / PL-Complexes with Various Compositions
[0057] The TF / PL-complexes are prepared as described in Example 1, but composed of various phospholipids in a concentration of 3.2 μM with 18 pM or 89 pM TF. The TF / PL-complexes are frozen in aliquots or lyophilized without sucrose with the lyophilization cycle described in Example 1.
TABLE 2Composition of TF / PL-complexesPL compositionPL concentrationTF concentration(weight ratio)(μM)(pM)PC:PS80 / 203.218PC:PS60 / 403.218PC:PS95 / 5 3.218PC:PS:PE78 / 17 / 5 3.218PC:PS:PE60 / 20 / 203.218PC:PS80 / 203.289PC:PS60 / 403.289PC:PS95 / 5 3.289PC:PS:PE78 / 17 / 5 3.289PC:PS:PE60 / 20 / 203.289
PC: 1,2-Dioleoyl-sn-glycero-3-phosphocholine (DOPC)
PS: 1-Palmitoyl-2-oleoyl-sn-glycero-3-phosphoserine (POPS)
PE: 1,2-Dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE)
[0058] Thrombin generation curves are measured as described above. The most characteristic parameter, the peak thrombin, i.e. the maximum t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com