Recombinant expression vector for human beta-NGF and recombinant cell strain containing same
A technology for expression vectors and eukaryotic cells, applied to cells modified by introducing foreign genetic material, recombinant DNA technology, and the use of vectors to introduce foreign genetic material. Antibody response and other issues, to achieve the effect of uniform drug resistance, high cell drug resistance, and uniform expression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
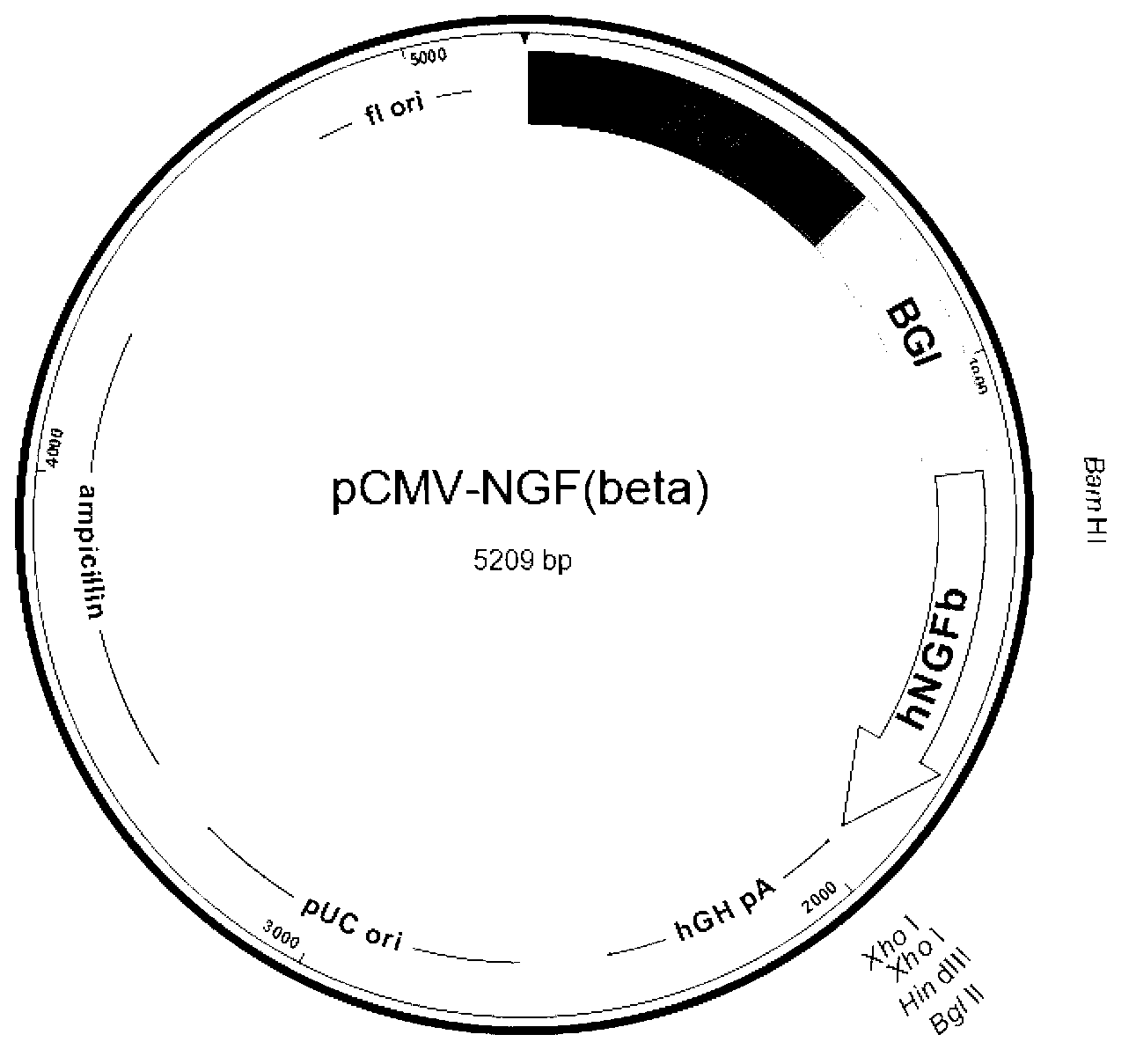
[0032] Example 1 Construction of β-NGF recombinant expression plasmid of the present invention
[0033] 1. Cloning of human β-NGF gene and construction of pCMV-β-NGF recombinant vector
[0034] (1) Total RNA was extracted from human placental cells using TriZol.
[0035] (2) Using the total RNA obtained in step (1) as a template, use Superscript III reverse transcriptase to synthesize a cDNA library by reverse transcription.
[0036] (3) Using the cDNA library obtained in step (2) as a template, use primers that specifically amplify the full length of the β-NGF gene, and perform PCR amplification under the catalysis of pfx DNA polymerase; the PCR reaction conditions are: 94°C pre-denaturation 3min; 30 cycles of denaturation at 94°C for 30s, annealing at 55°C for 30s, and extension at 68°C for 1min.
[0037]The upstream and downstream primers for specifically amplifying the full length of the β-NGF gene are respectively shown in SEQ ID No.4 and SEQ ID No.5. The upstream and d...
Embodiment 2
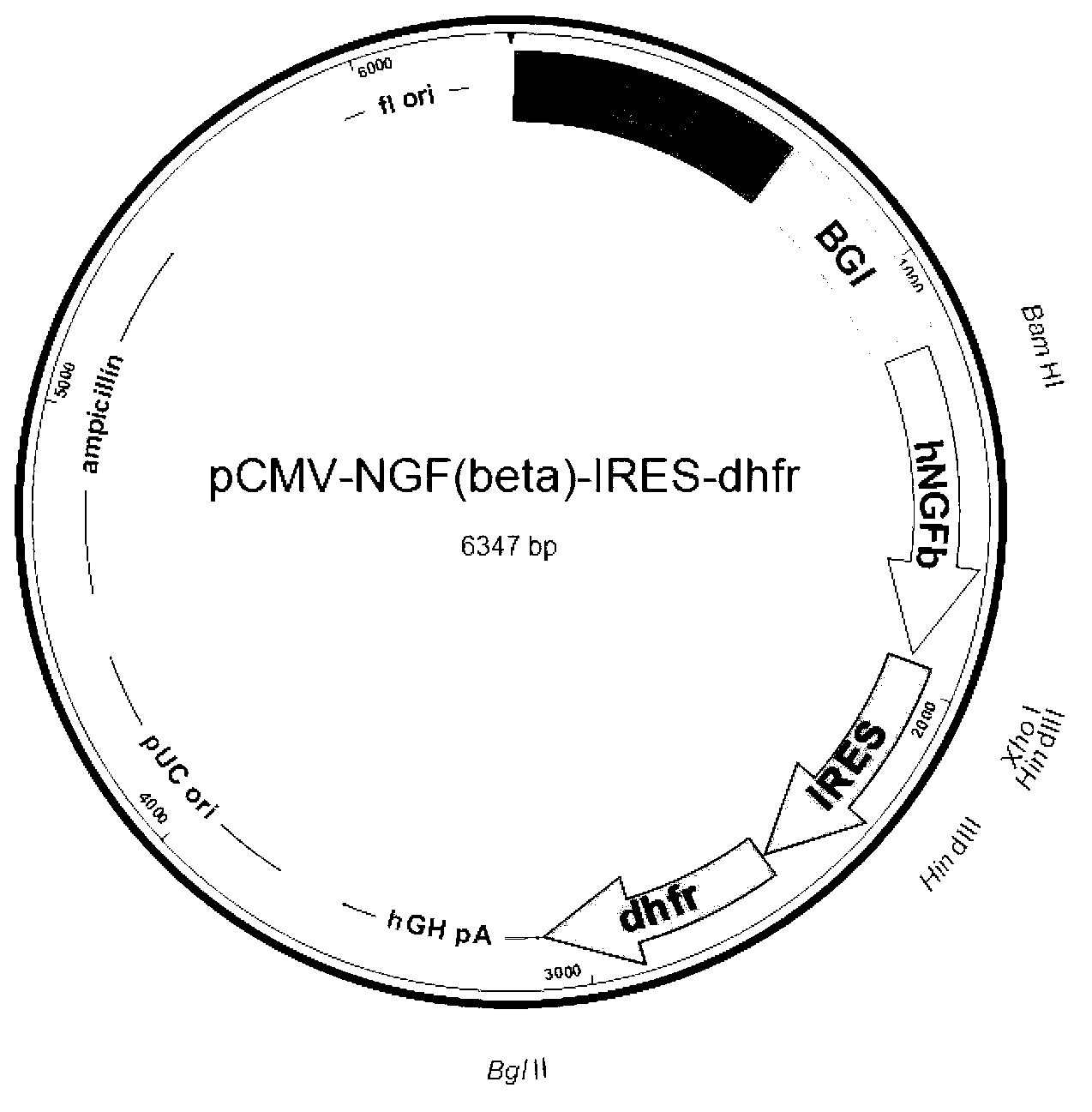
[0049] Example 2 Construction of β-NGF recombinant cell line of the present invention
[0050] 1. Transfection and stable screening of NIH293 cells
[0051] (1) Plasmid purification: The pCMV-β-NGF-IRES-dhfr plasmid prepared in Example 1 was purified by cesium chloride density gradient centrifugation and kept for future use.
[0052] (2) Transfection and screening of recombinant cell lines: Use DEME complete medium (containing 10% calf serum) at 37°C, 5% CO 2 NIH293 cells were cultured to a 60% monolayer under the environment of NIH293 cells, and the above-mentioned purified pCMV-β-NGF-IRES-dhfr plasmid was transfected into NIH293 cells by calcium phosphate co-precipitation method; after 24 hours, the medium was changed, and 50nM MTX was added to filter. After the cells adapt to the selection pressure, the concentration of MTX is gradually increased, which is 100nM, 200nM, 400nM and 800nM. After the cells are adapted to 800nM MTX, further increase the MTX concentration to 1...
Embodiment 3
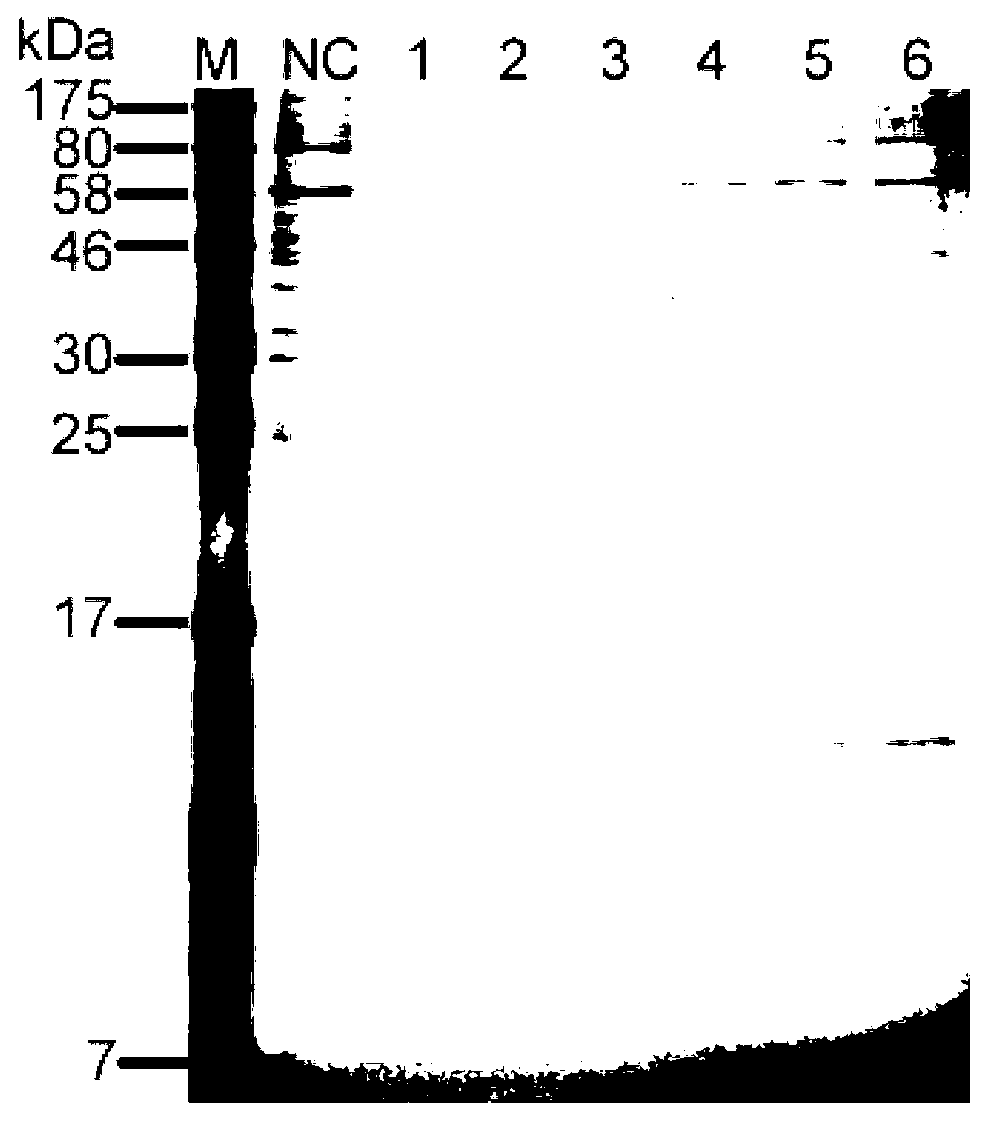
[0056] Example 3 Identification of β-NGF secreted by the β-NGF recombinant cell line of the present invention
[0057] A β-NGF recombinant cell line obtained in Example 2 was cultured without serum, the medium was collected and the protein was concentrated to obtain β-NGF, and the β-NGF was identified by mass spectrometry.
[0058] Mass spectrometric identification method:
[0059] The obtained β-NGF was separated by capillary liquid chromatography-electrospray ionization-quadrupole-time-of-flight mass spectrometry (UPLC-ESI-Q-TOF-MS) for peptide map determination: β-NGF was hydrolyzed with trypsin and dissolved in 0.1% (v / v) formic acid solution was analyzed by reversed-phase nanoliter liquid chromatography-electrospray tandem mass spectrometry. Using BEH130 capillary liquid chromatography, load 10 μl of the sample to the enrichment column (180 μm×20mm Symmetry C18trap column), desalt with buffer A (that is, 0.1% formic acid aqueous solution) at a flow rate of 10 μl / min for ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| bleeding rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com