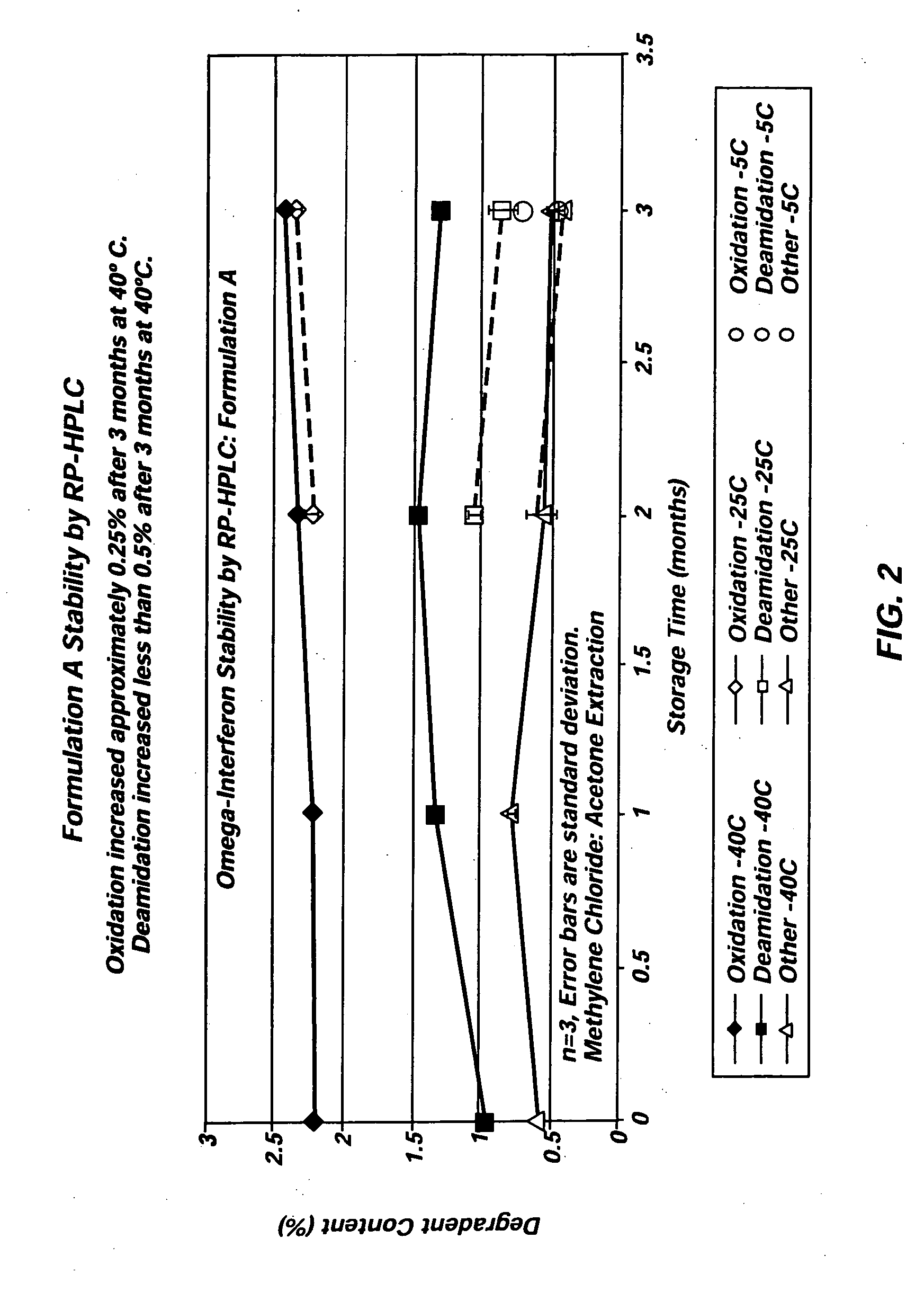
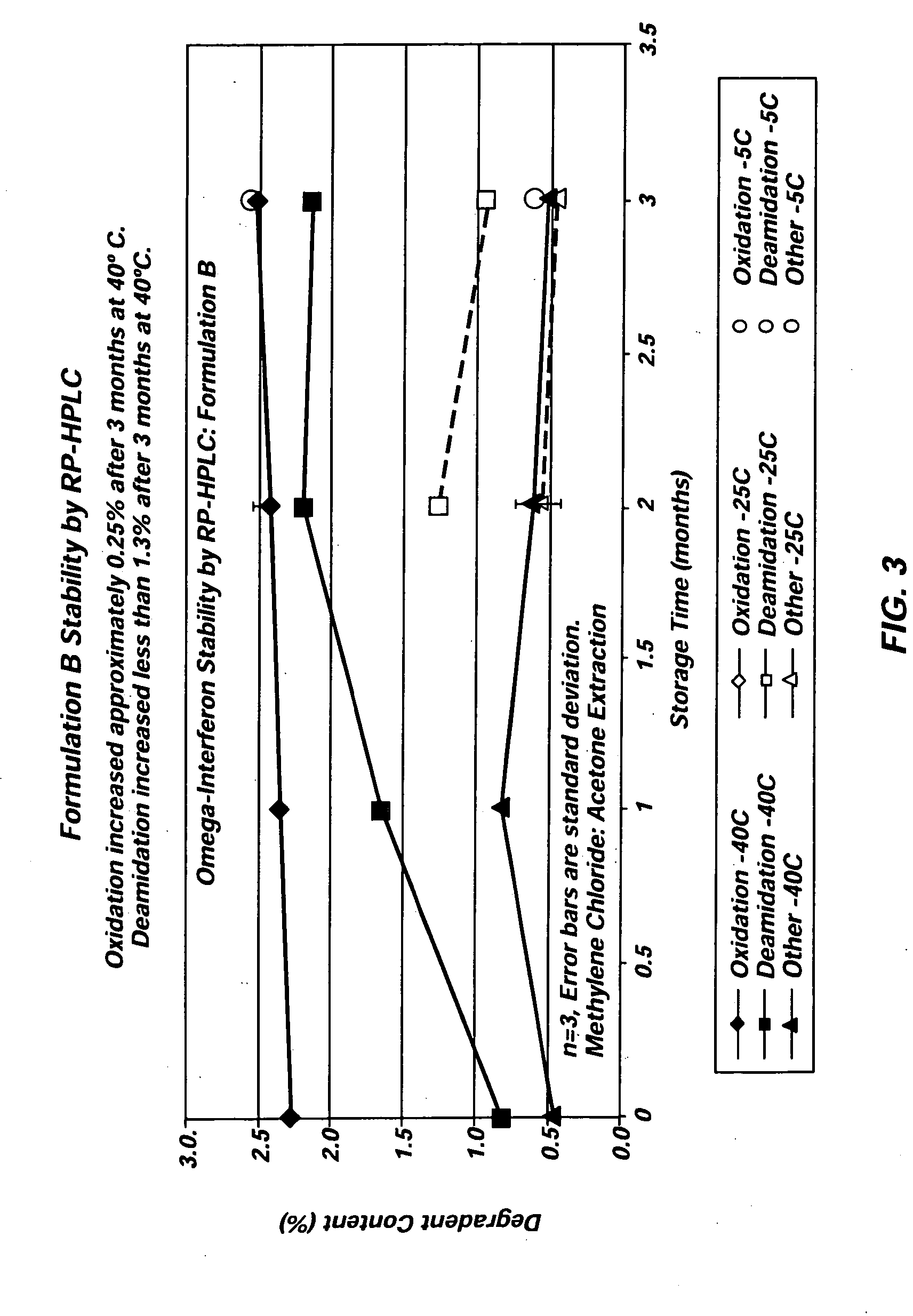
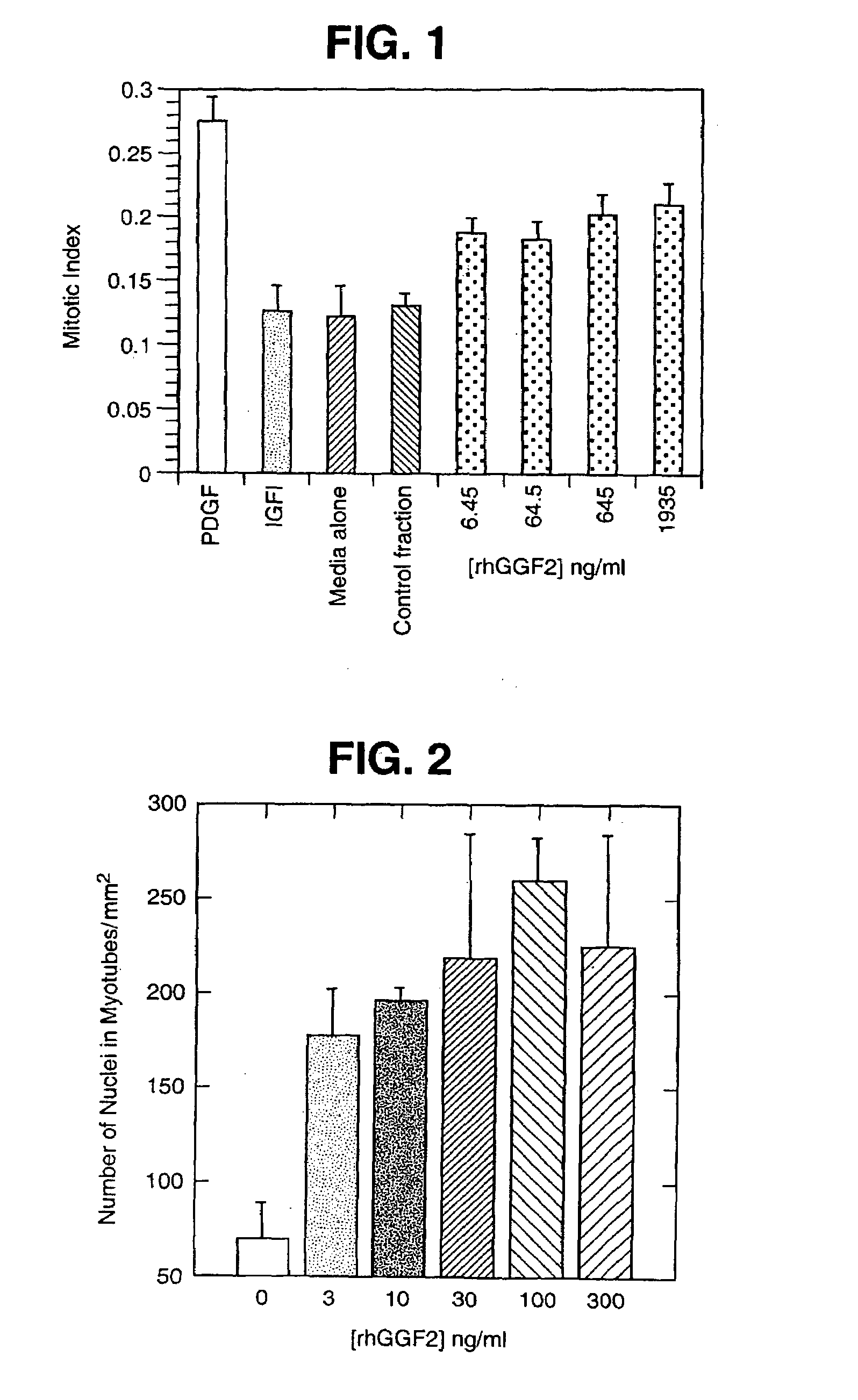
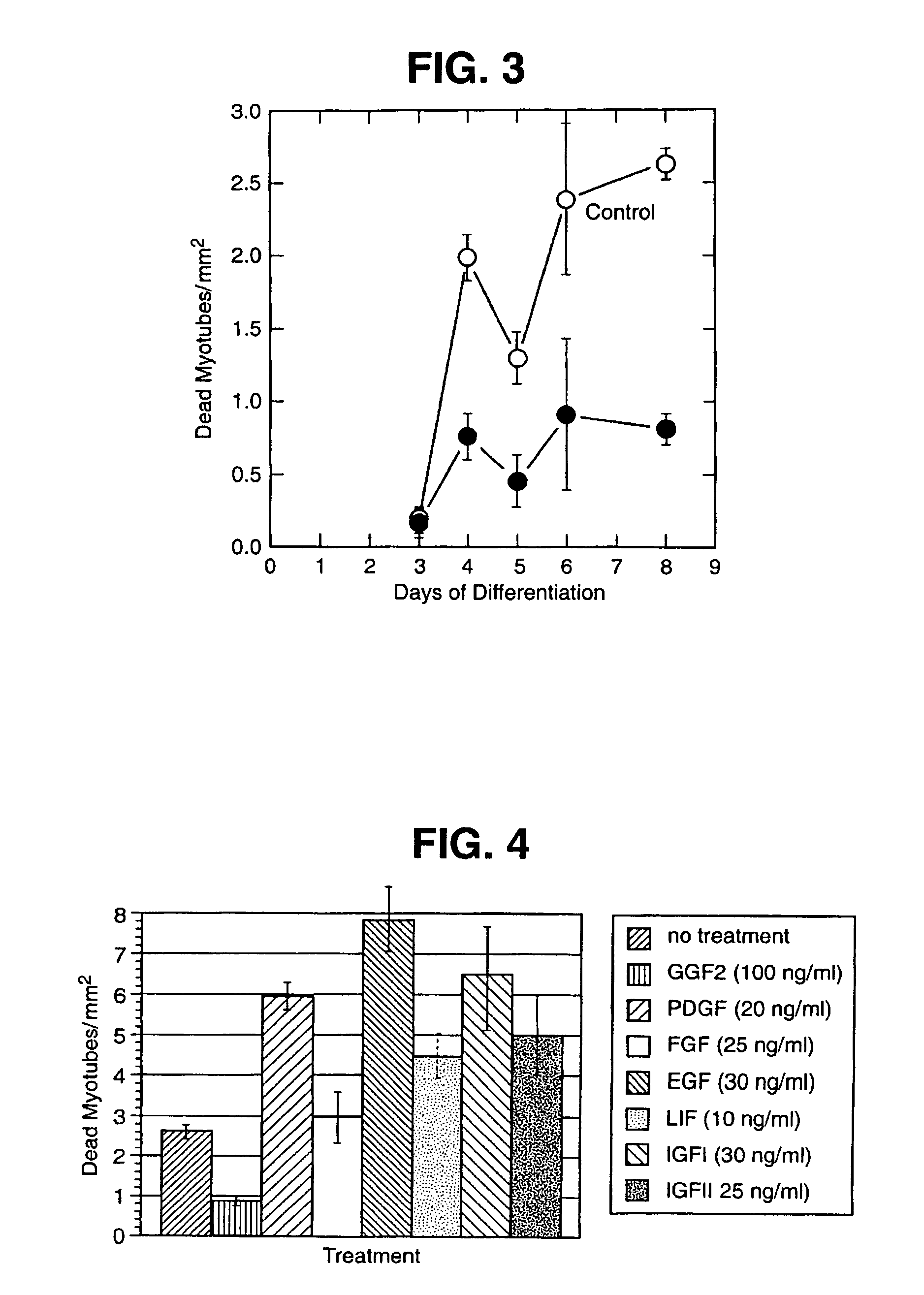
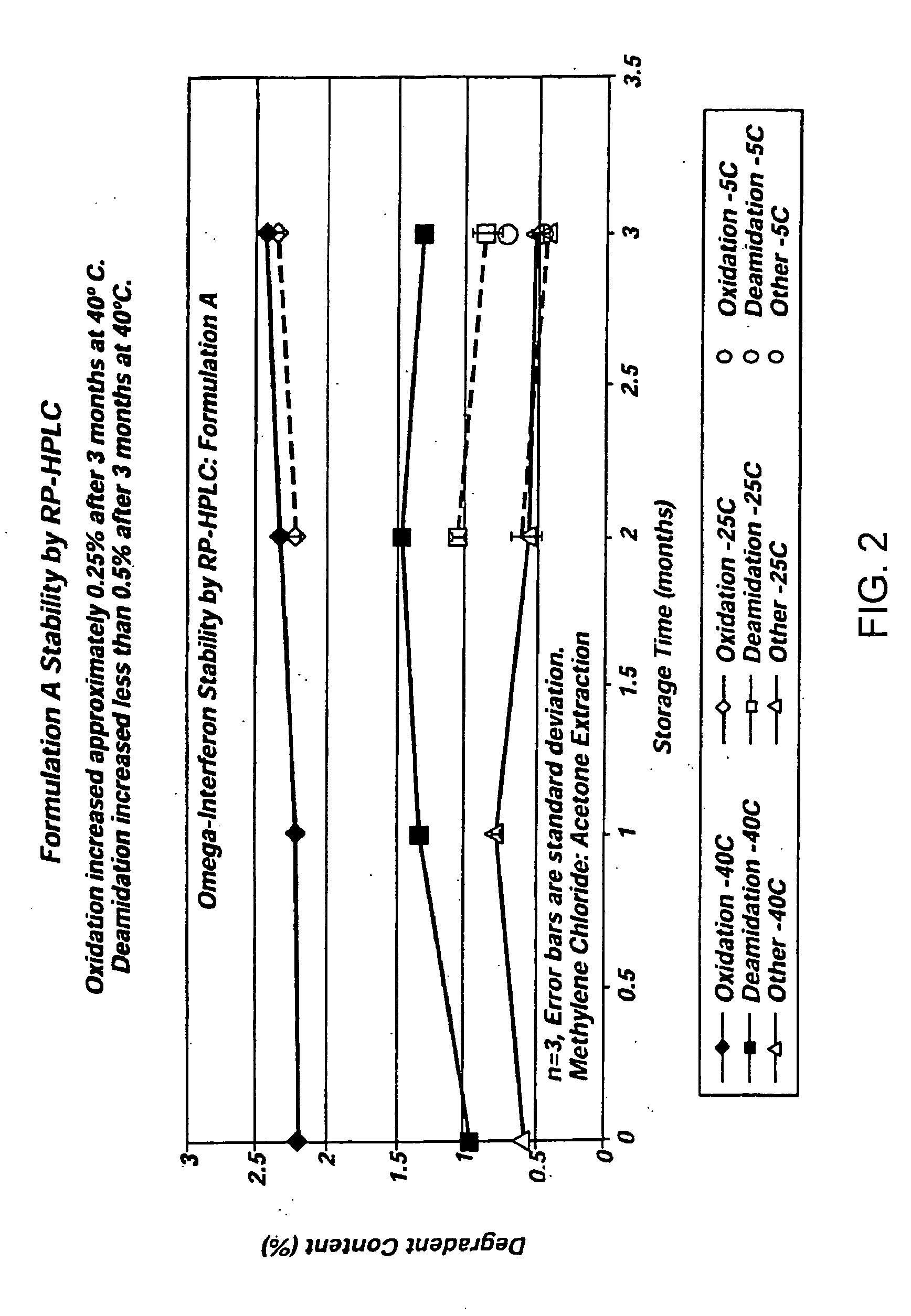
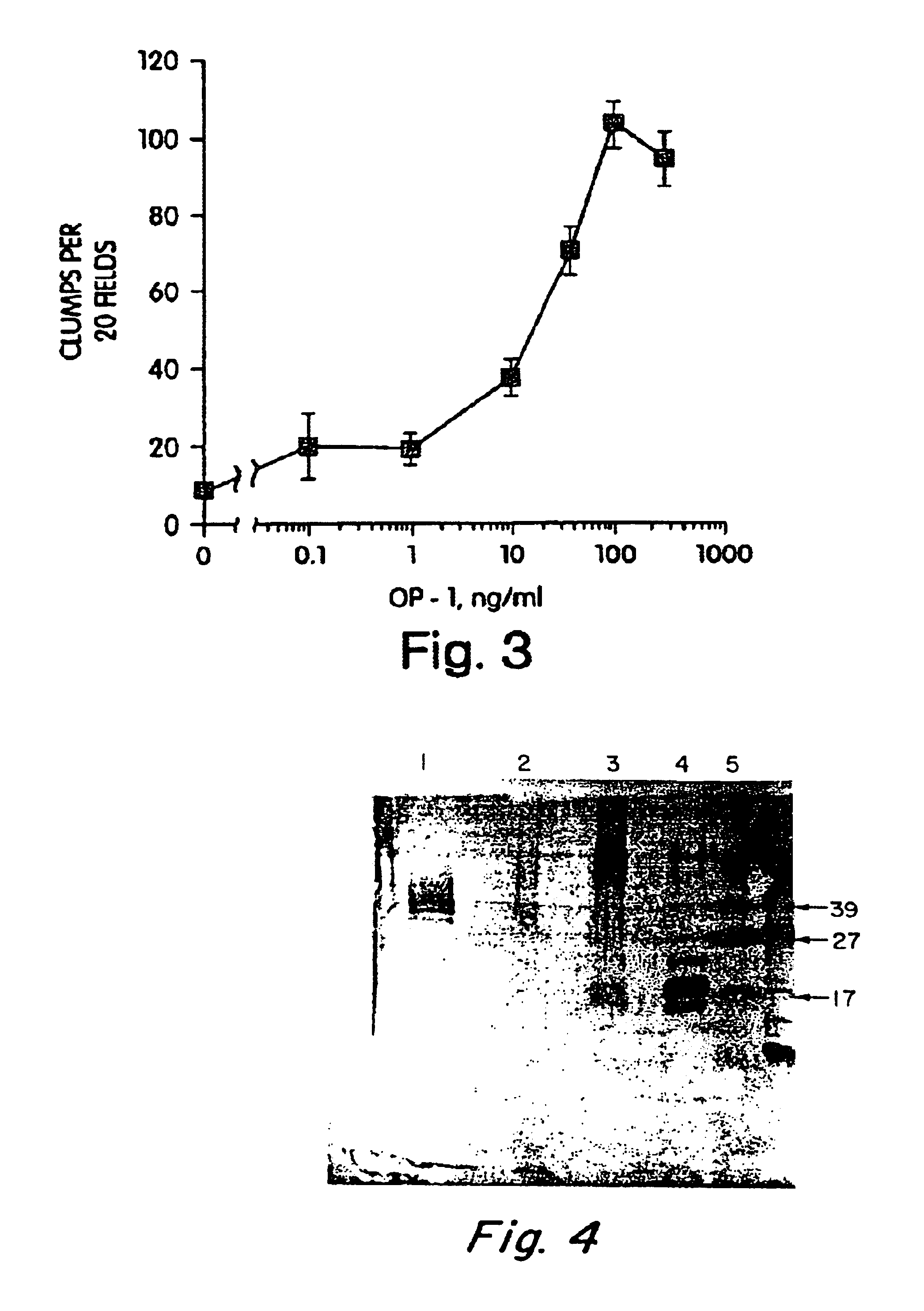
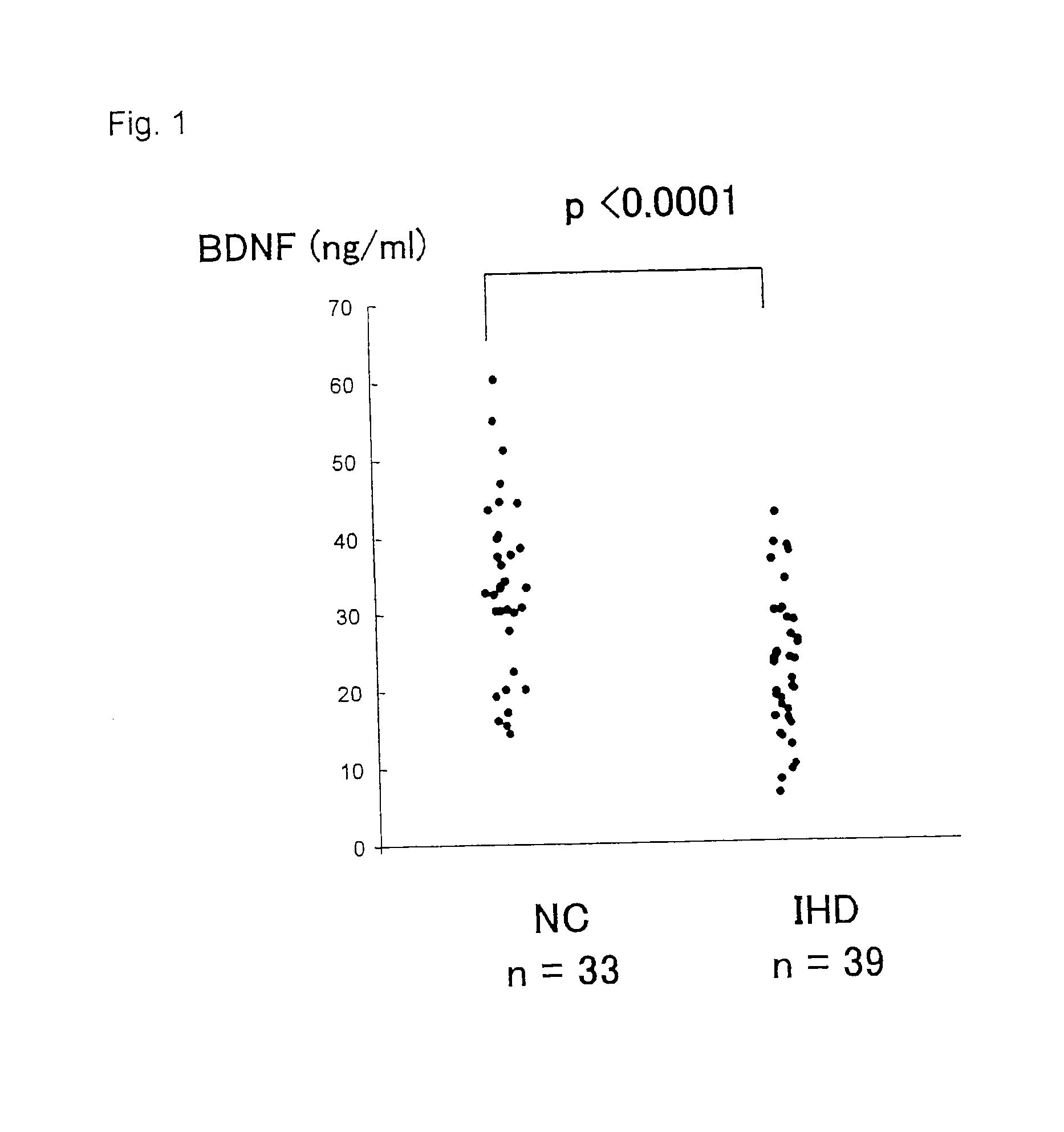Patents
Literature
409results about "Nerve growth factors" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Methods of using and compositions comprising immunomodulatory compounds for treatment and management of macular degeneration
InactiveUS20040091455A1Extension of timeUseful in treatingBiocideSenses disorderActive agentSurgical procedures
Methods of treating, preventing and / or managing macular degeneration are disclosed. Specific embodiments encompass the administration of an immunomodulatory compound, or a pharmaceutically acceptable salt, solvate, hydrate, stereoisomer, clathrate, or prodrug thereof, alone or in combination with a second active agent and / or surgery. Pharmaceutical compositions, single unit dosage forms, and kits suitable for use in methods of the invention are also disclosed.
Owner:CELGENE CORP
Dispersible macromolecule compositions and methods for their preparation and use
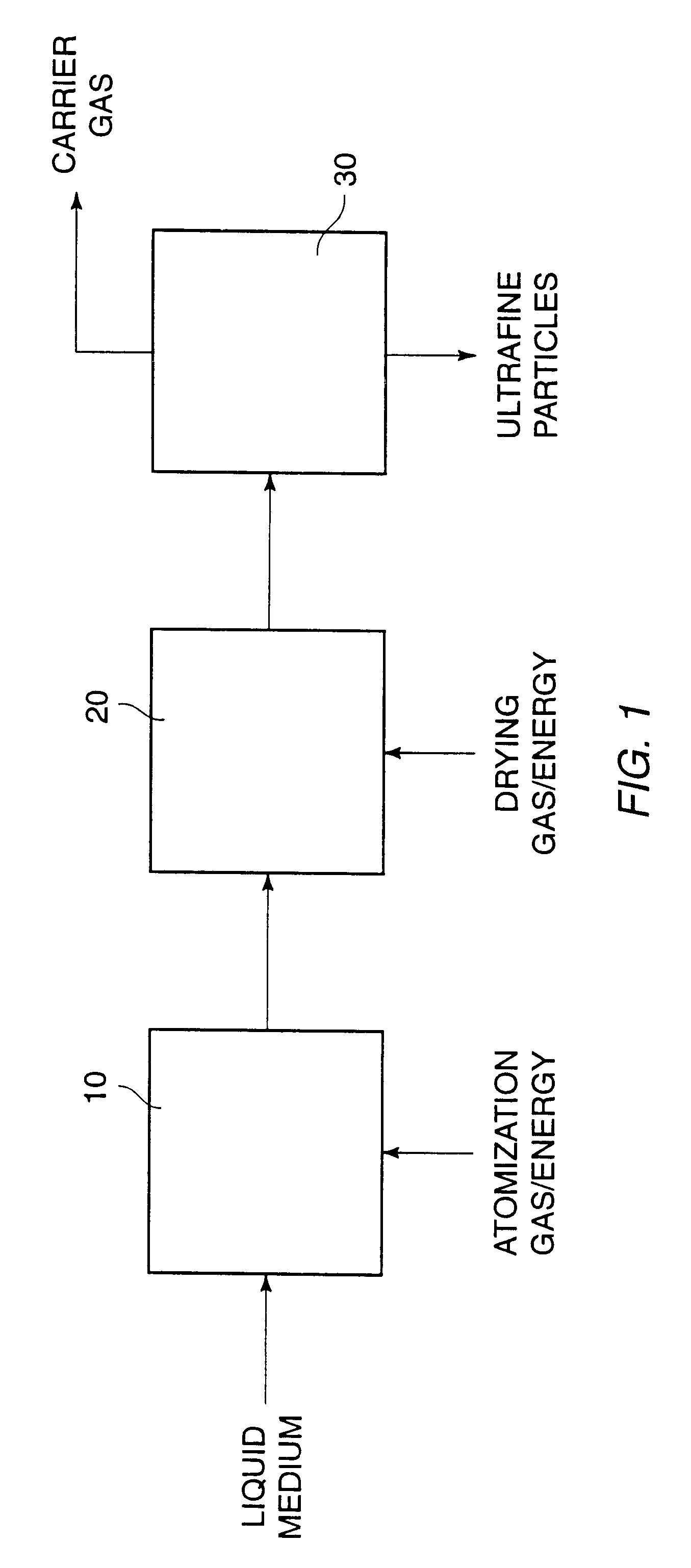
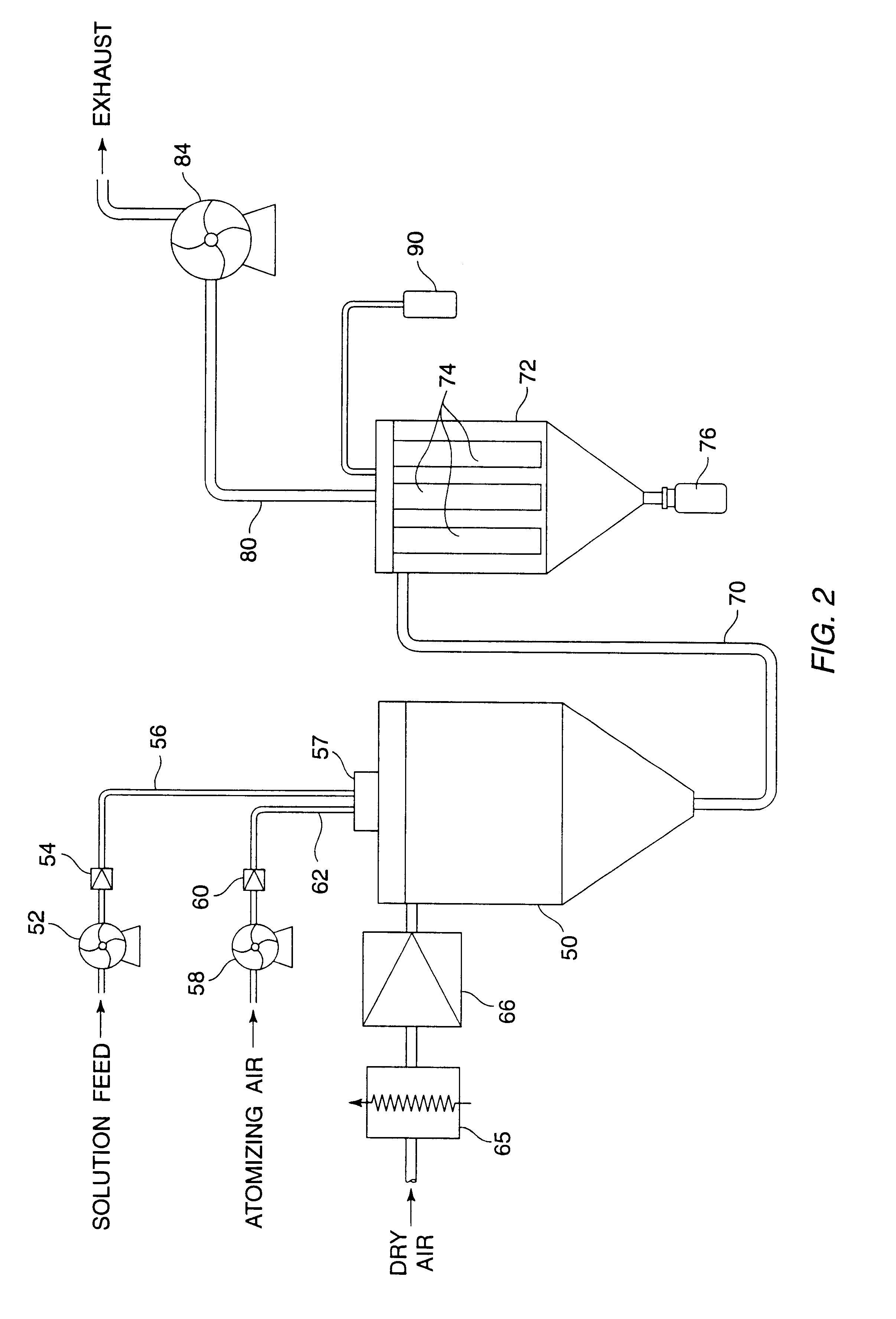
A process for preparing ultrafine powders of biological macromolecules comprises atomizing liquid solutions of the macromolecules, drying the droplets formed in the atomization step, and collecting the particles which result from drying. By properly controlling each of the atomization, drying, and collection steps, ultrafine dry powder compositions having characteristics particularly suitable for pulmonary delivery for therapeutic and other purposes may be prepared.
Owner:NOVARTIS FARMA
Clostridial toxin derivatives able to modify peripheral sensory afferent functions
InactiveUS6395513B1Pain reliefReduce and preferably prevent transmissionNervous disorderPeptide/protein ingredientsClostridial toxinProjection neuron
The invention relates to an agent specific for peripheral sensory afferents. The agent may inhibit the transmission of signals between a primary sensory afferent and a projection neuron by controlling the release of at least one neurotransmitter or neuromodulator from the primary sensory afferent. The agent may be used in or as a pharmaceutical for the treatment of pain, particularly chronic pain.
Owner:HEALTH PROTECTION AGENCY +1
C-aryl glucoside SGLT2 inhibitors and method
Owner:BRISTOL MYERS SQUIBB CO
Administration of growth factors for the treatment of CNS disorders
ActiveUS20070254842A1Prevents and delay onsetReduce severityHeavy metal active ingredientsSenses disorderDiseaseNervous system
A method and system that is directed to the local delivery of growth factors to the mammalian CNS to treat CNS disorders associated with neuronal death and / or dysfunction is described.
Owner:RGT UNIV OF CALIFORNIA
Non-aqueous single phase vehicles and formulations utilizing such vehicles
InactiveUS20050008661A1Increase stickinessReduce occlusionPowder deliveryPeptide/protein ingredientsEngineeringSolvent
The present invention includes materials and methods for providing vehicles useful for providing drug formulations that address the potential drawbacks of known nonaqueous formulations. In particular, the present invention includes nonaqueous vehicles that are formed using a combination of polymer and solvent that results in a vehicle that is miscible in water. The nonaqueous vehicles facilitate the formulation of drug formulations that are stable over time, even when stored at, or exposed to, elevated temperatures. Moreover, the miscible vehicles of the present invention allow the preparation of drug formulations that work to reduce the occurrence of partial or complete occlusions of the delivery conduits included in delivery devices used to administer the drug formulations.
Owner:DURECT CORP
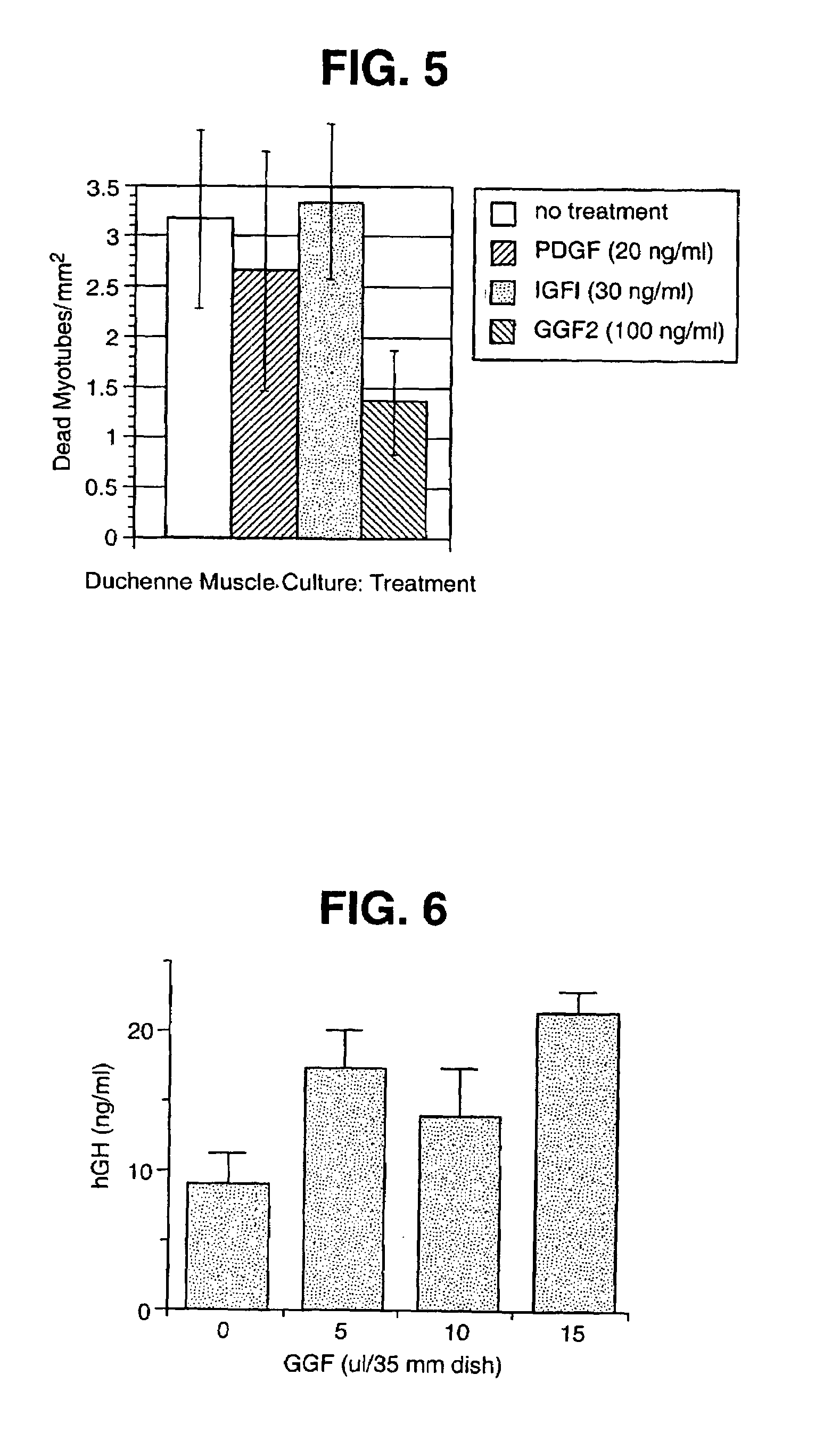
Methods for treating muscle diseases and disorders
InactiveUS7037888B1Reduce the amount of solutionQuality improvementNervous disorderPeptide/protein ingredientsSmooth muscleDisease
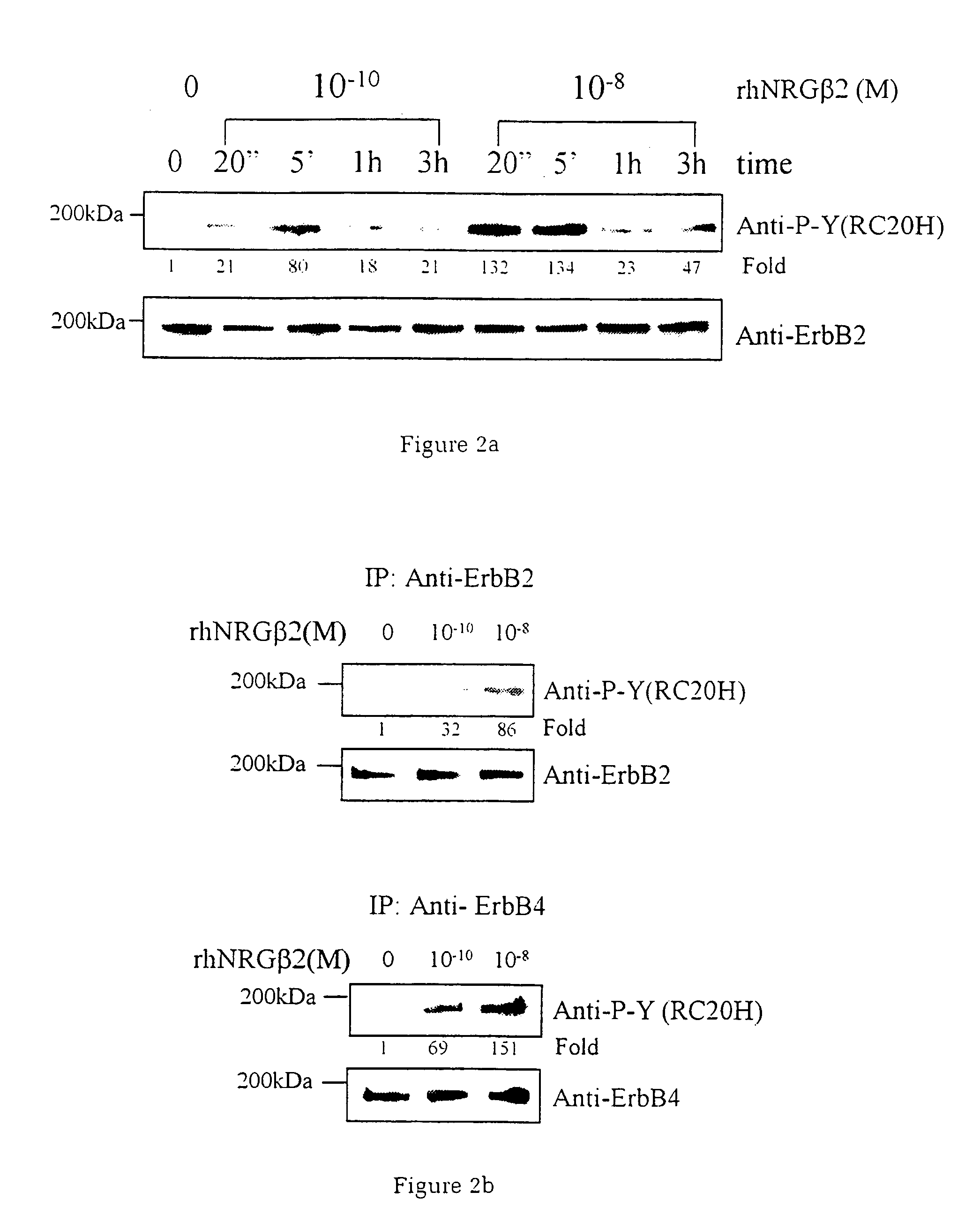
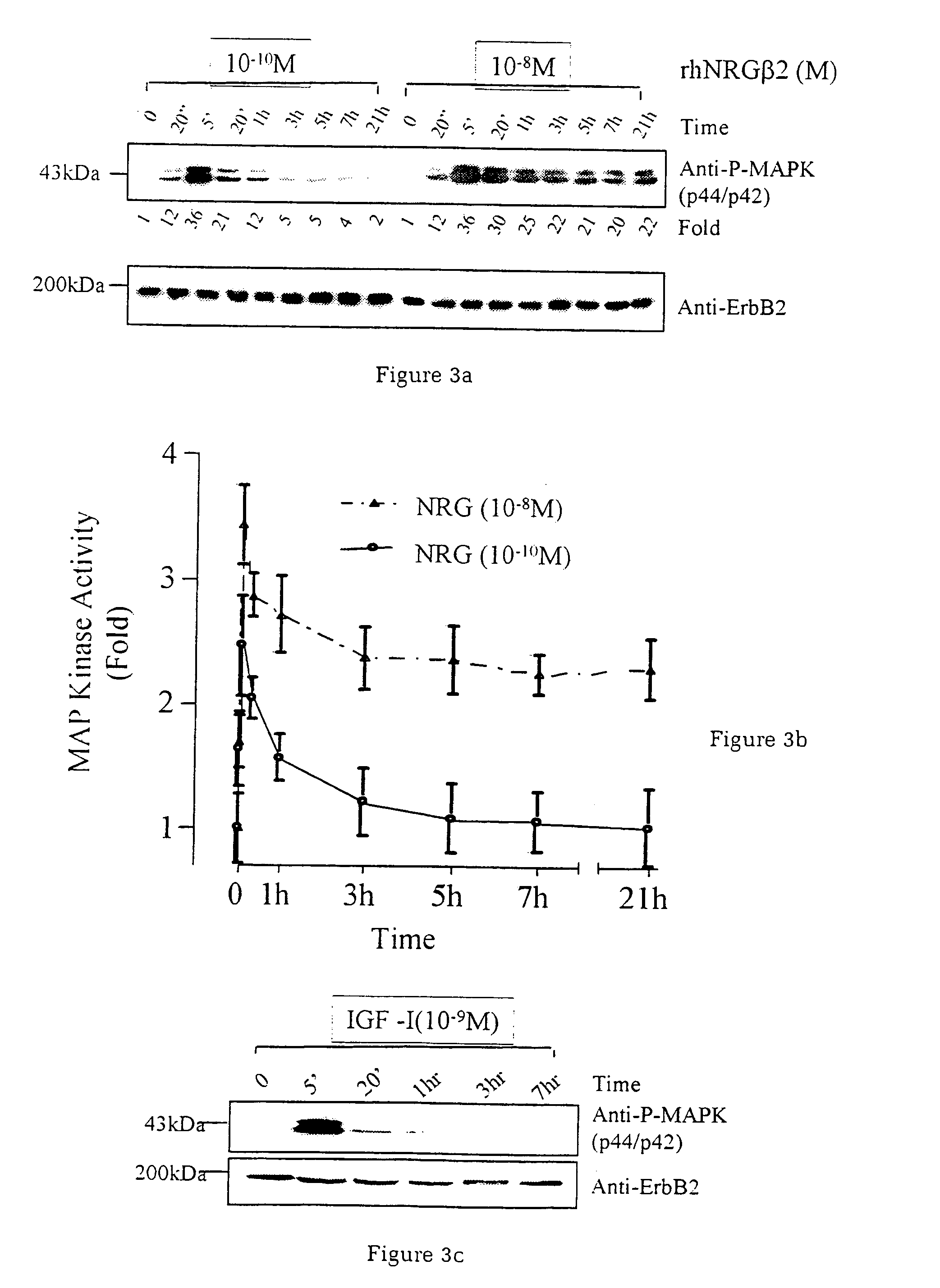
The invention relates to methods of treating diseases and disorders of the muscle tissues in a vertebrate by the administration of compounds which bind the p185erbB2 receptor. These compounds are found to cause increased differentiation and survival of cardiac, skeletal and smooth muscle.
Owner:ACORDA THERAPEUTICS INC
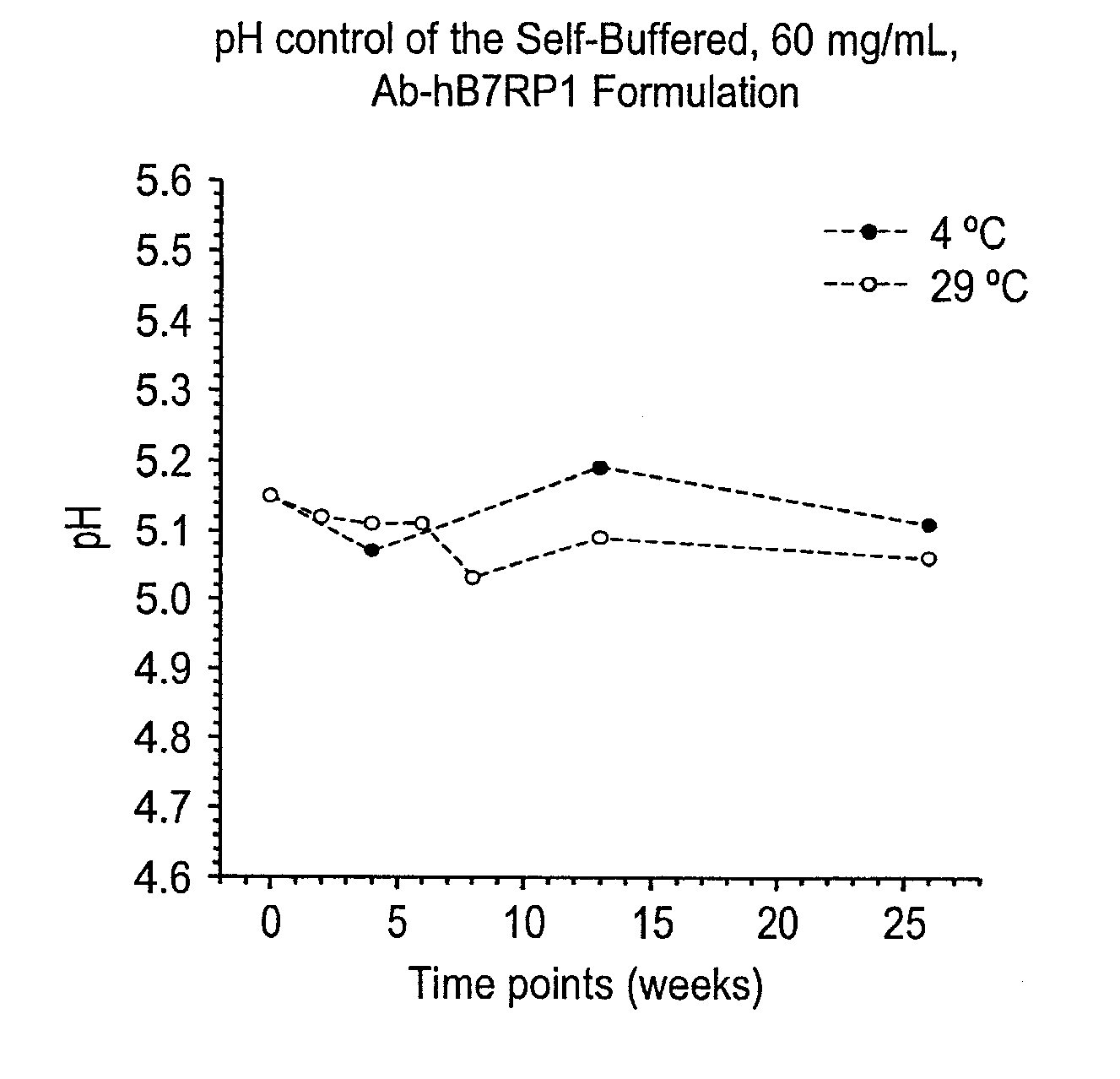
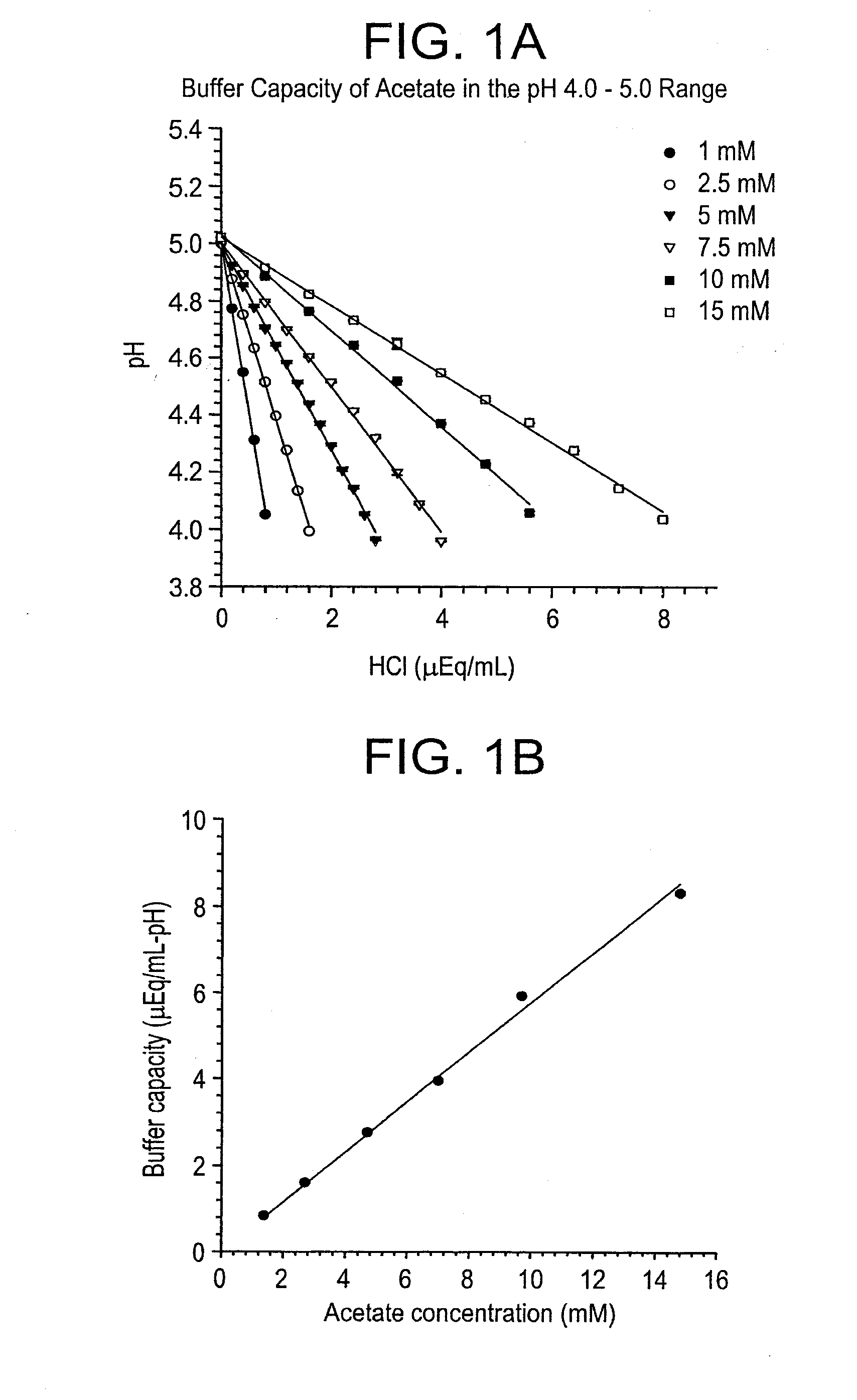
Self-Buffering Protein Formulations
InactiveUS20080311078A1Peptide/protein ingredientsInorganic non-active ingredientsDiseaseHuman medicine
The invention herein described, provides, among other things, self-buffering protein formulations. Particularly, the invention provides self-buffering pharmaceutical protein formulations that are suitable for veterinary and human medical use. The self-buffering protein formulations are substantially free of other buffering agents, stably maintain pH for the extended time periods involved in the distribution and storage of pharmaceutical proteins for veterinary and human medical use. The invention further provides methods for designing, making, and using the formulation. In addition to other advantages, the formulations avoid the disadvantages associated with the buffering agents conventionally used in current formulations of proteins for pharmaceutical use. The invention in these and other respects can be productively applied to a wide variety of proteins and is particularly useful for making and using self-buffering formulations of pharmaceutical proteins for veterinary and medical use, especially, in particular, for the treatment of diseases in human subjects.
Owner:AMGEN INC
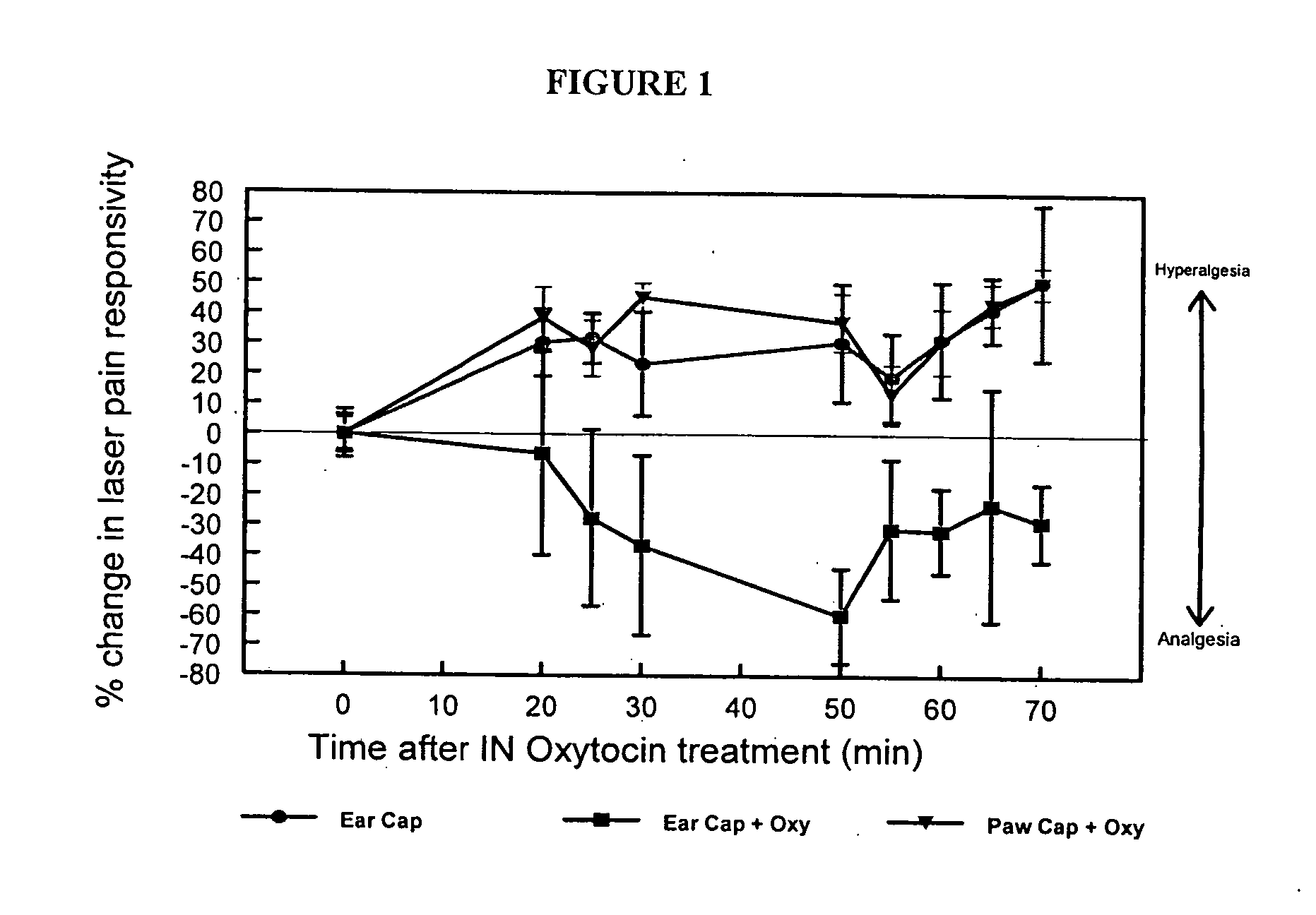
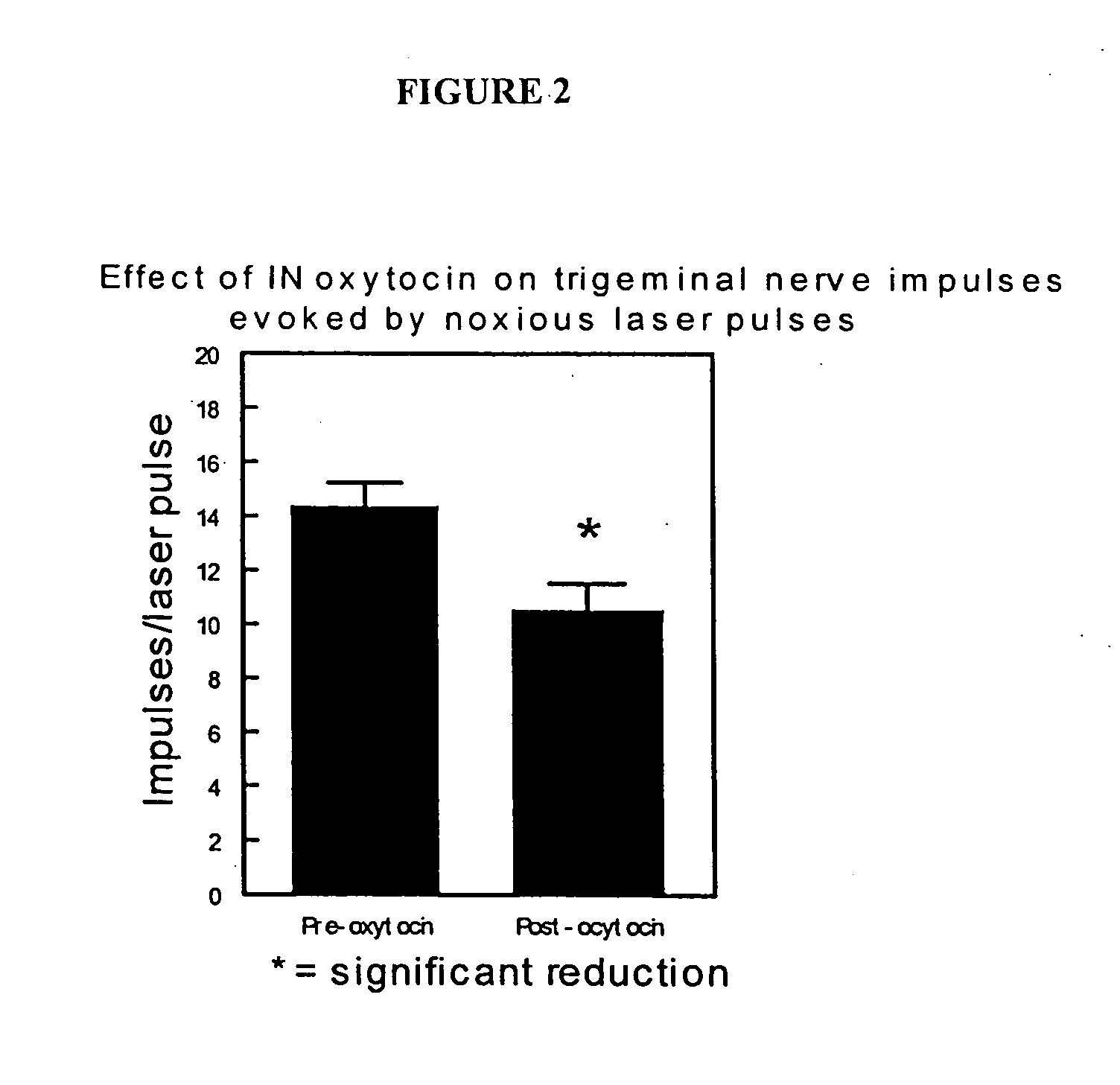
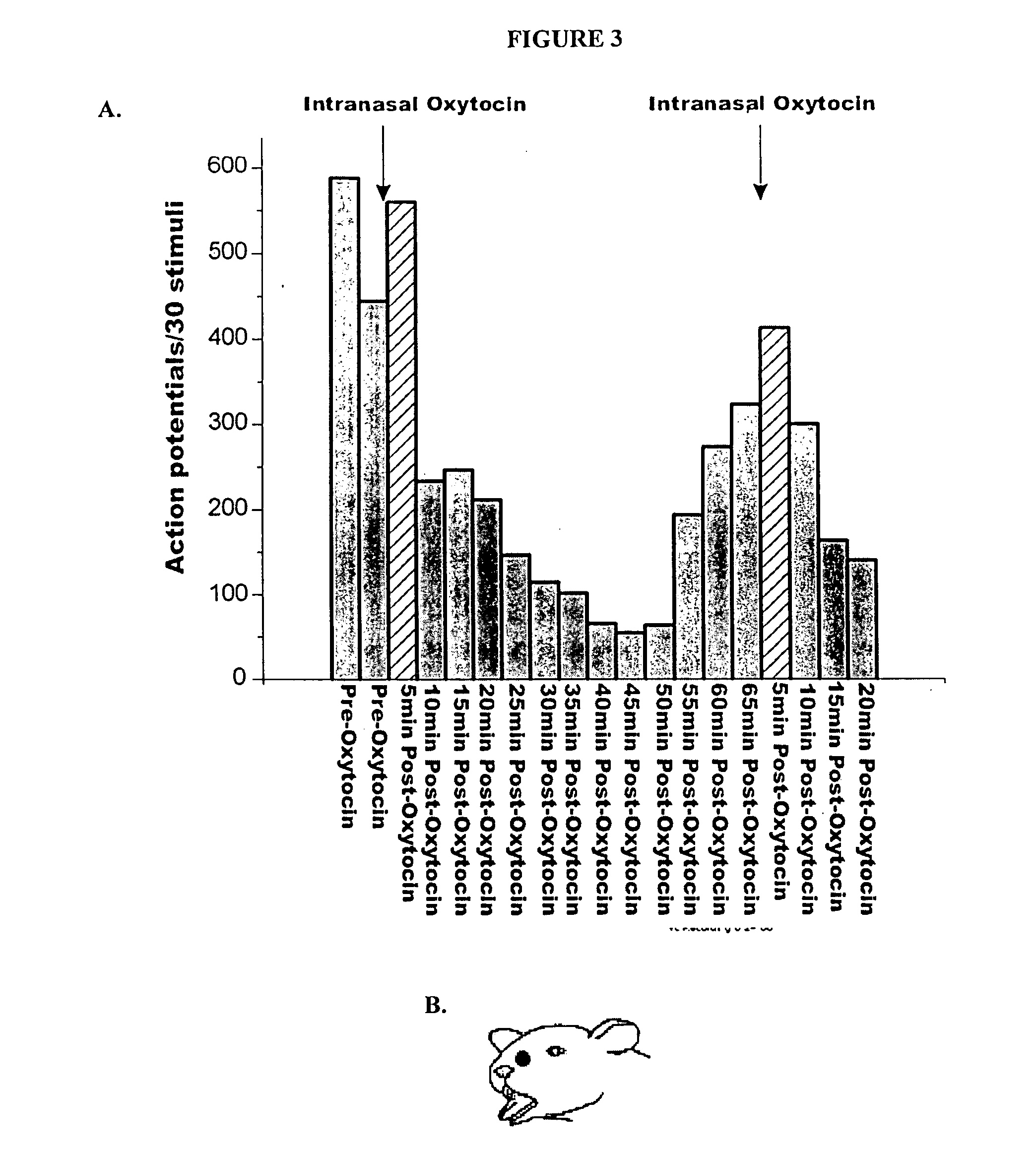
Methods for treatment of headaches by administration of oxytocin
InactiveUS20070054843A1Reduction of pain ratingReduce probabilityOrganic active ingredientsNervous disorderHeadache DisordersTrigeminal neuralgia
The present invention relates to methods for the treatment of headache and headache disorders. The methods comprise administration of an oxytocin peptide for the treatment of primary and secondary headaches or trigeminal neuralgia.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV +2
Biodegradable scaffolds and uses thereof
InactiveUS20060002978A1Strengthen cellsEnhanced tissue growthPowder deliveryPeptide/protein ingredientsBiodegradable scaffoldPolymer chemistry
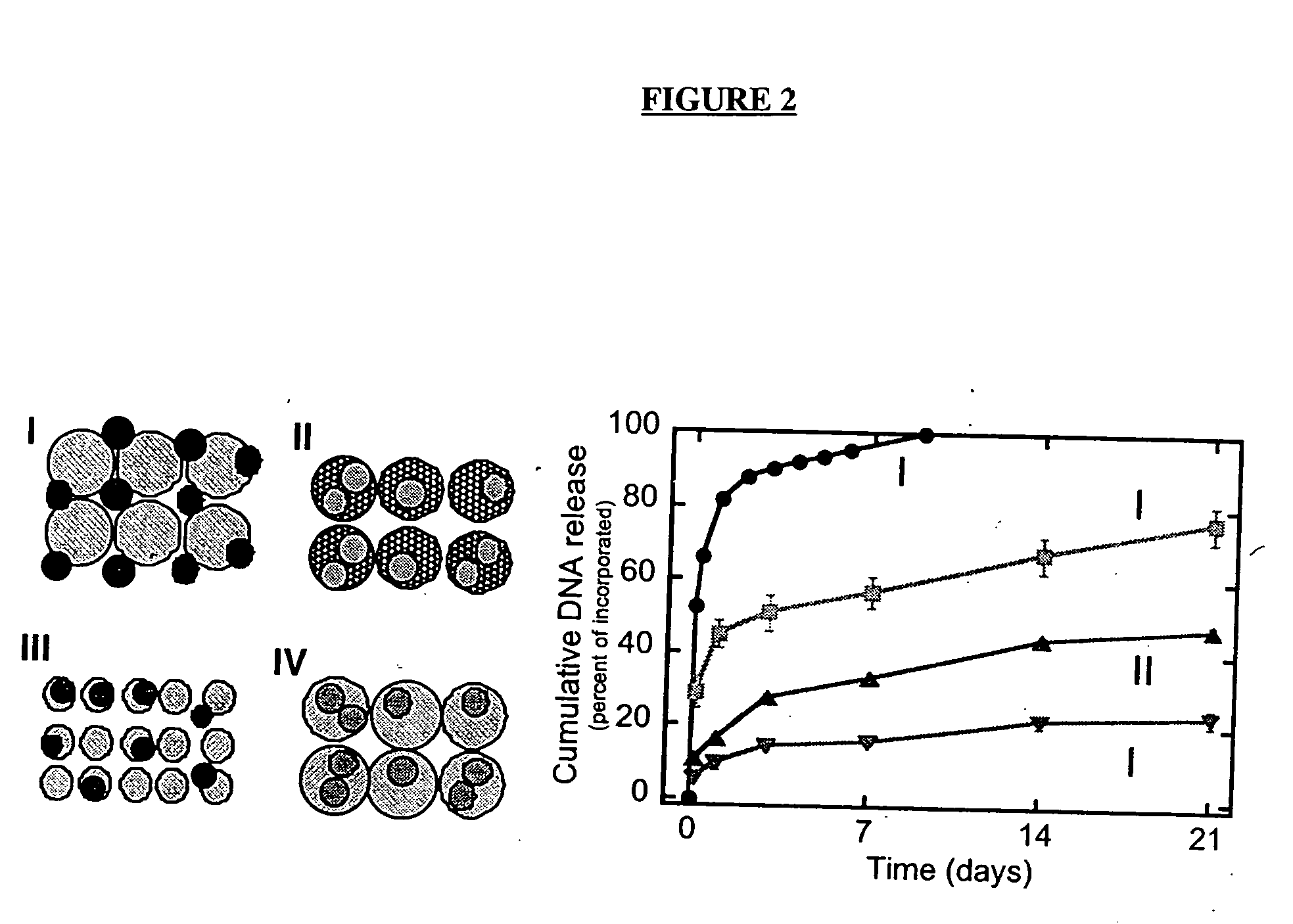
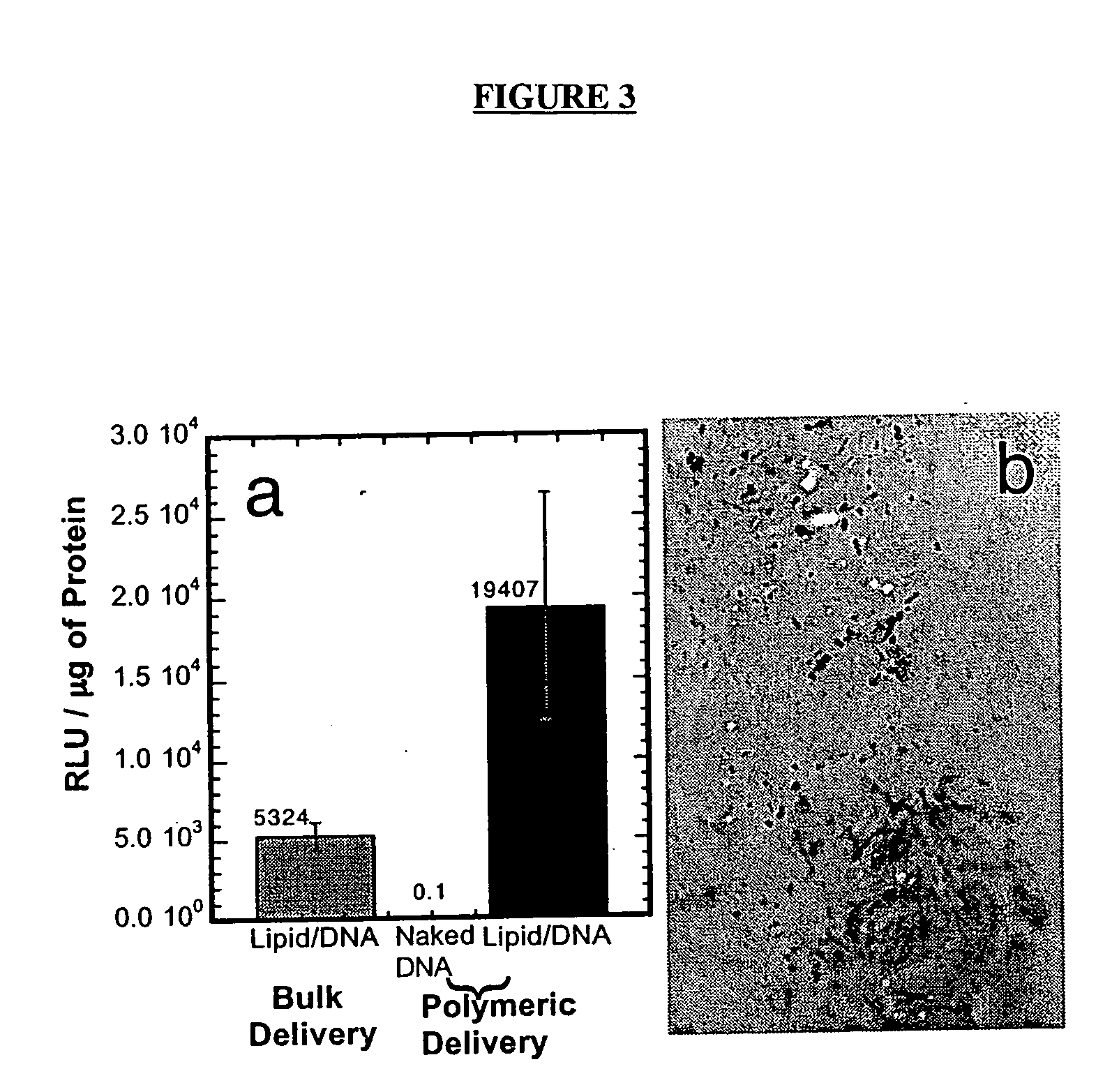
The invention is directed to scaffolds containing porous polymer material prepared by a process of gas foaming / particulate leaching and a wet granulation step prior to gas foaming and particulate leaching, particularly having a characteristic interconnected pore structure, as well as sustained release of protein, DNA or cells, and to methods for using such porous polymer material for preparation of scaffolds, particularly for tissue engineering.
Owner:NORTHWESTERN UNIV
Non-aqueous single phase vehicles and formulations utilizing such vehicles
InactiveUS20050276856A1Decrease in levelLower Level RequirementsPowder deliveryPeptide/protein ingredientsMedicineDelivery vehicle
Owner:DURECT CORP
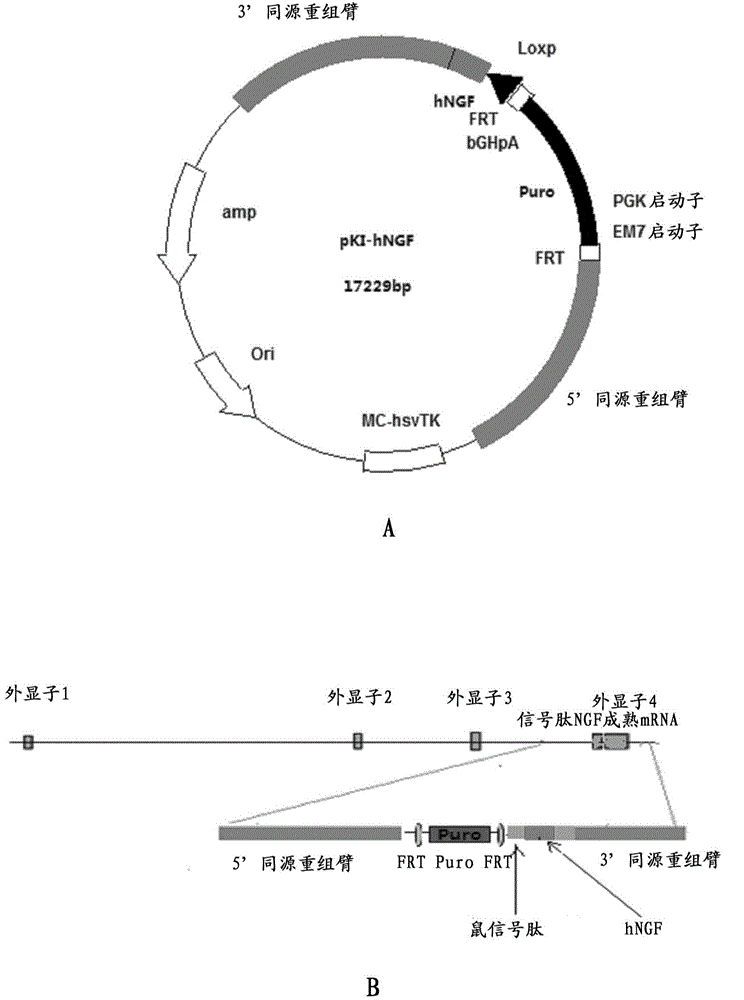
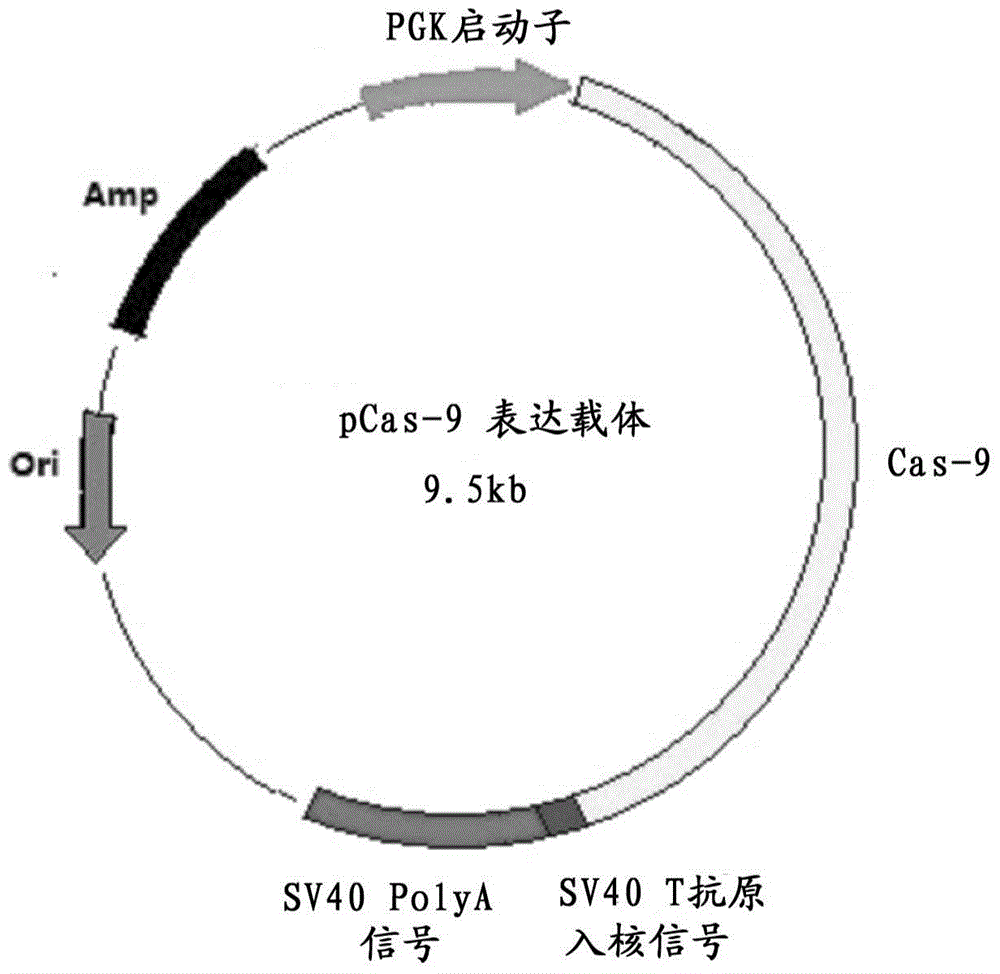
Preparation method for transgenic mice capable of producing human nerve growth factor
ActiveCN104561095AEasy to operateIncrease success rateAnimals/human peptidesVector-based foreign material introductionPlasmid dnaGenetically modified mouse
The invention discloses a method for producing transgenic mice through a homologous recombination technology. The method comprises the following steps: replacing mouse NGF genes on mouse chromosomes by human NGF genes through a Cas-9 / CRISPR gene knock-in technology, and obtaining the genes with homozygous human NGF genes through breeding and knocking the genes in mice, thus obtaining filial generation mice with salivary glands capable of secreting human NGF. Compared with the conventional targeting technology utilizing positive and negative double screening homologous recombination genes, the method disclosed by the invention is simple to operate, capable of being realized only by transfecting mouse embryonic stem cells by three plasmid DNAs or carrying out pronucleus injection on the zygotes of mice, and high in success rate which is up to 2-5% and remarkably higher than the mouse embryonic stem cell positive rate of 0.1% of the common gene targeting technology.
Owner:深圳市国创纳米抗体技术有限公司
Fusion proteins for blood-brain barrier delivery
ActiveUS20070081992A1Avoid significant immunogenic reactionAvoid significant reactionSenses disorderNervous disorderMediated transportBlood–brain barrier
The invention provides compositions, methods, and kits for increasing transport of agents across the blood brain barrier while allowing their activity once across the barrier to remain substantially intact. The agents are transported across the blood brain barrier via one or more endogenous receptor-mediated transport systems. In some embodiments the agents are therapeutic, diagnostic, or research agents.
Owner:ARMAGEN TECH +1
Cardiac muscle function and manipulation
InactiveUS7226907B1Augment PE-mediated cardiac muscle cell differentiationImprove heart functionPeptide/protein ingredientsGenetic material ingredientsCardiomyocyte growthNeuregulin
Owner:ZENSUN (SHANGHAI) SCI & TECH CO LTD
Cytomodulating peptides and methods for treating neurological disorders
InactiveUS20040186052A1Reducing neural cell deathReduce cell deathNervous disorderPeptide/protein ingredientsNeuritisNeuro degeneration
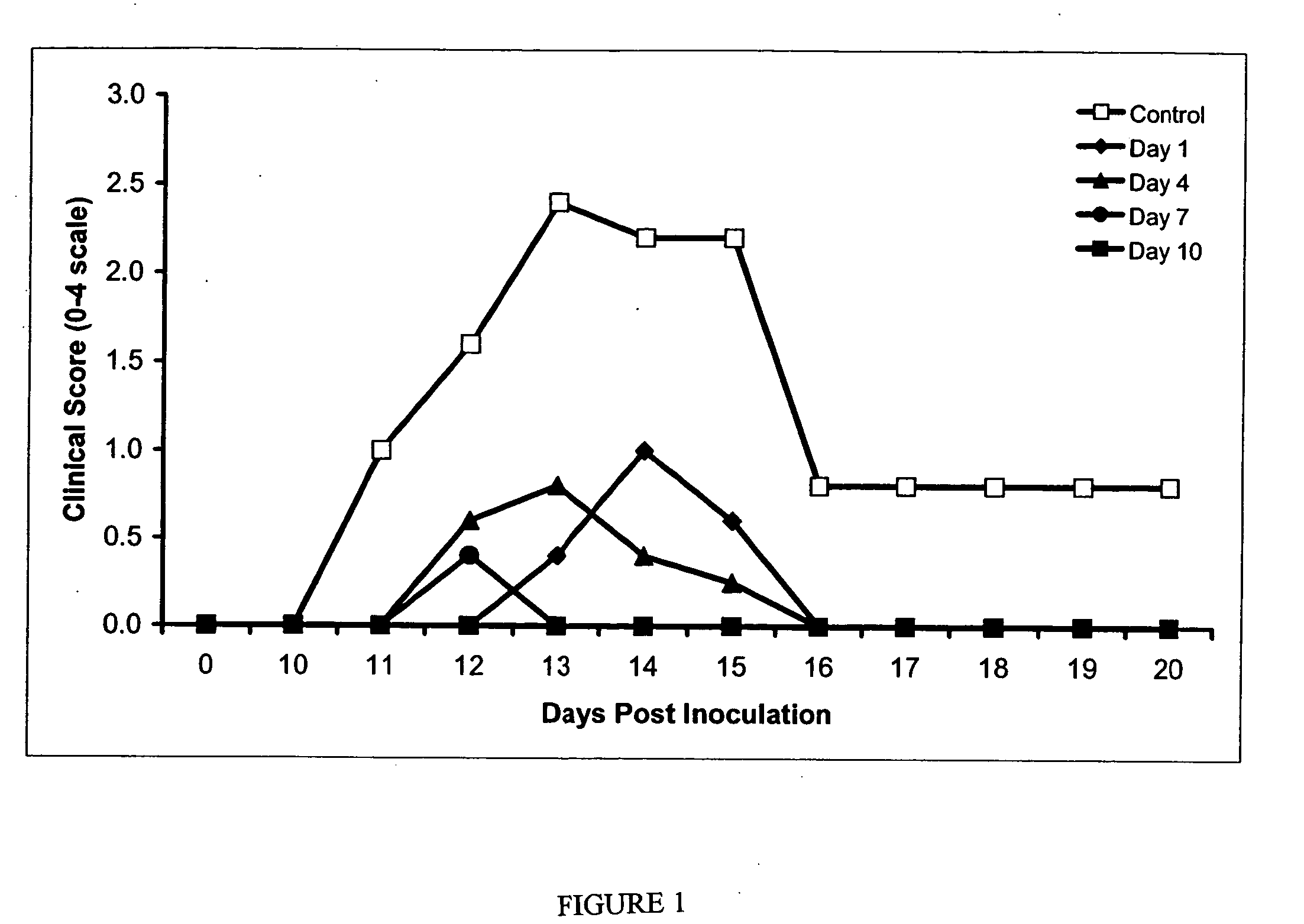
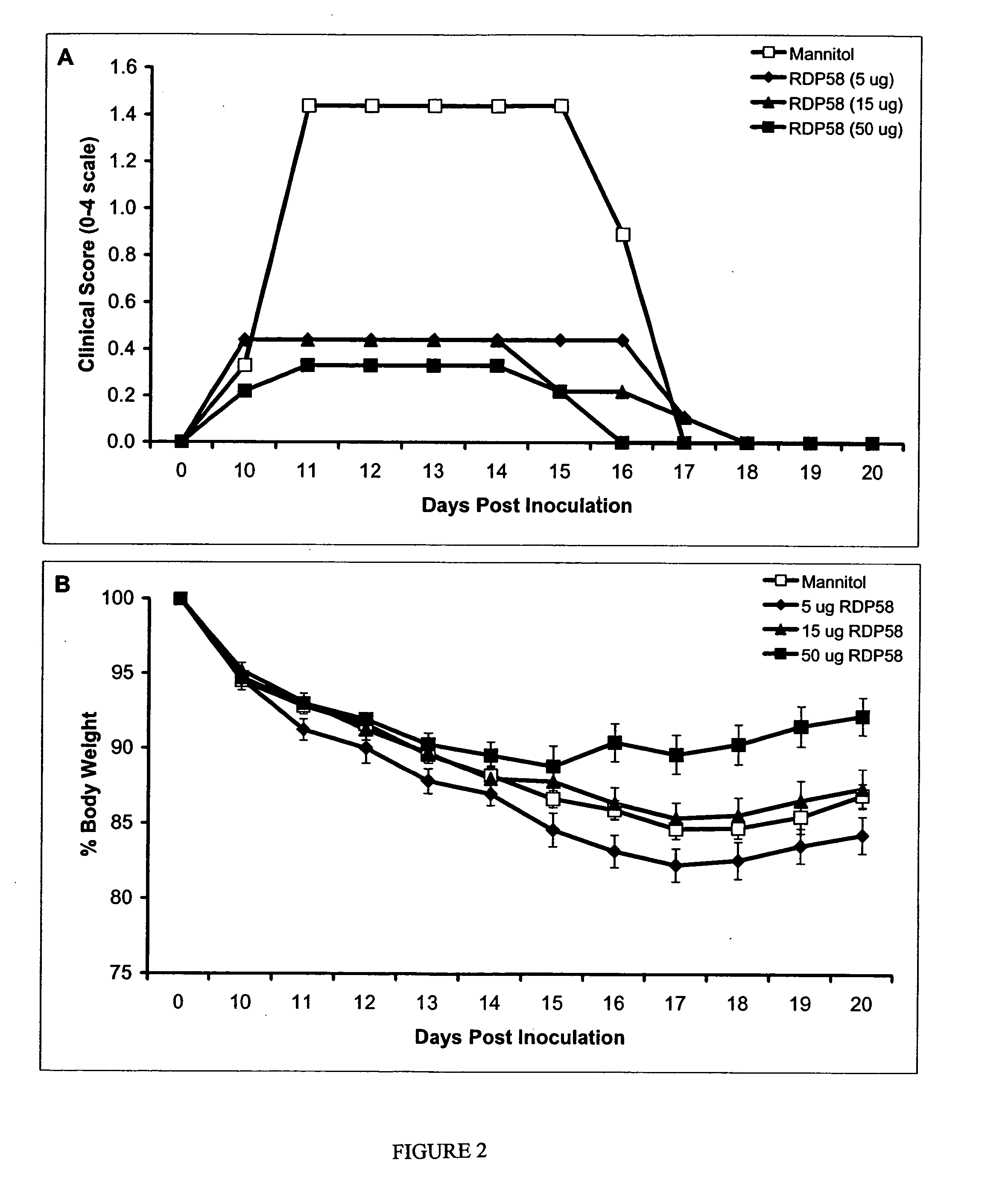
Compositions and methods are provided for inhibiting neuronal cell death and the loss of neuronal contacts resulting from acute and chronic neurological disorders, including neurodegenerative and neuroinflammatory diseases. The subject compositions and methods utilize RDP-58 compositions capable of providing a direct neuroprotective effect on neuronal cells in conjunction with the inhibition of autoimmune and inflammatory processes.
Owner:SANGSTAT MEDICAL
Biodegradable ocular implants and methods for treating ocular conditions
InactiveUS20080089923A1Improve storage characteristicsAvoid rapid degradationAntibacterial agentsBiocideDiseaseOphthalmology
Owner:SURMODICS INC
Β-turn peptidomimetic cyclic compounds
InactiveUS6881719B2Easy to manageReduce deliveryOrganic active ingredientsTetrapeptide ingredientsDiseaseNeurotrophin Receptor p75
Proteolytically stable small molecule β-turn peptidomimetic compounds have been identified as agonists or antagonists of neurotrophin receptors, such as TrkA. A compound of particular interest binds the immunoglobulin-like C2 region of the extracellular domain of TrkA, competes the binding of another TrkA ligand, affords selective trophic protection to TrkA-expressing cell lines and neuronal primary cultures, and induces the differentiation of primary neuronal cultures. The small β-turn peptidomimetic compounds of the invention can activate a tyrosine kinase neurotrophin receptor that normally binds a relatively large protein ligand. Such compounds that bind the extracellular domain of Trk receptors are useful pharmacological agents to address disorders where Trk receptors play a role, by targeting populations selectively.
Owner:TEXAS A&M UNIVERSITY +1
Self-assembling peptides for regeneration and repair of neural tissue
The present invention provides methods and compositions for enhancing regeneration and / or repair of neural tissue. One method include providing a nanoscale structured material at the site of injury, wherein the nanoscale structured material provides an environment that is permissive for regeneration of neural tissue and allows axon growth from a location on one side of a site of injury or barrier to a location on the other side of the site of injury or barrier. A second method includes introducing a composition comprising self-assembling peptides into the subject at the site of injury, wherein the peptides are amphiphilic peptides that comprise substantially equal proportions of hydrophobic and hydrophilic amino acids and are complementary and structurally compatible. A variety of compositions comprising a nanoscale structured material or precursor thereof, and an additional substance such as a regeneration promoting factor, are also provided. In certain embodiments of the invention the nanoscale structured material or precursor thereof comprises self-assembling peptides. The invention further provides compositions and methods for repair of an intervertebral disc, including nucleus pulpusos repair.
Owner:MASSACHUSETTS INST OF TECH
Clostridial toxin derivatives able to modify peripheral sensory afferent functions
InactiveUS20030049264A1Pain reliefReduce and preferably prevent transmissionNervous disorderHydrolasesClostridial toxinMedicine
The invention relates to an agent specific for peripheral sensory afferents. The agent may inhibit the transmission of signals between a primary sensory afferent and a projection neutron by controlling the release of at least one neurotransmitter or neuromodulator from the primary sensory afferent. The agent may be used in or as a pharmaceutical for the treatment of pain, particularly chronic pain.
Owner:HEALTH PROTECTION AGENCY +1
Trophic factor combinations for nervous system treatment
The present invention relates to a composition including an effective amount of at least one of an antimicrobial peptide and a substance having an antimicrobial peptide effect and an effective amount of a neurotrophin. The composition can also include an effective amount of at least one of a growth factor and a neuropeptide. The present invention also relates a method of treating an injury to a nervous system of an animal that includes the steps of identifying the injury to the nervous system and applying to the injury an effective amount of at least one of antimicrobial peptide and a substance having an antimicrobial peptide effect. The method can also include applying an effective amount of one or more trophic factors selected from the group consisting of a growth factor, a neurotrophin, and a neuropeptide to the injury.
Owner:WISCONSIN ALUMNI RES FOUND
Matrix composed of a naturally-occurring protein backbone cross linked by a synthetic polymer and methods of generating and using same
A method of treating a disorder characterized by tissue damage is provided. The method comprising providing to a subject in need-thereof a composition which comprises a synthetic polymer attached to denatured fibrinogen or a therapeutic portion of the fibrinogen, the composition being formulated for releasing the therapeutic portion of the fibrinogen in a pharmacokinetically regulated manner, thereby treating the disorder characterized by tissue damage or malformation.
Owner:REGENTIS BIOMATERIALS
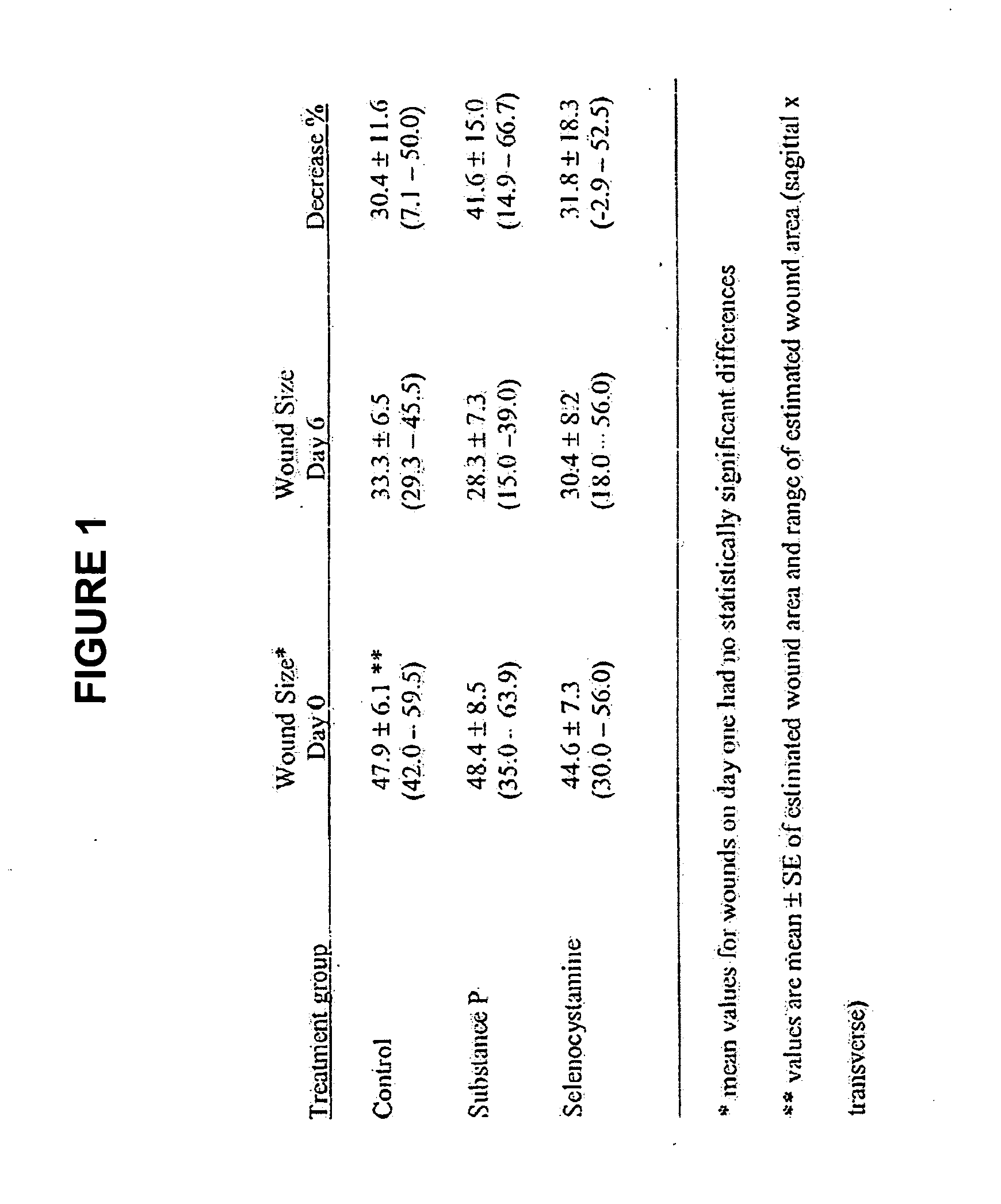
Methods and compositions using Substance P to promote wound healing
InactiveUS20070154448A1Promote healingGrowth promoting activityOrganic active ingredientsBiocideSubstance KMammalian tissue
Healing of wounds in mammalian tissue may be enhanced by the application of certain neuropeptides, optionally in combination with known growth promoting hormones. Exemplary neuropeptides include tachykinins, such as Substance P, Substance K, and the like, as well as calcitonin gene-related peptides. The compositions may further include a polymeric delivery carrier and are utilized by applying to the site of the wound. Wounds may be vascular or avascular wounds. The compositions promote elaboration of cellular matrices and development of cellular attachment mechanisms in addition to stimulating cellular proliferation.
Owner:AUXANO BIOLOGICS
Morphogen-induced dendritic growth
InactiveUS6949505B1Alleviate neural pathway damageAlleviate and inhibit immunologically related responseBiocideNervous disorderMammalNeurotrophic factors
Disclosed are methods and compositions for maintaining neural pathways in a mammal including: enhancing survival of neurons at risk of dying; inducing cellular repair of damaged neurons and neural pathways; stimulating neurons to maintain their differentiated phenotype; and promoting dendritic outgrowth, including maintaining dendritic arbors and regenerating dendritic architecture. In one embodiment, the invention provides means for stimulating CAM expression in neurons. The invention also provides means for evaluating the status of nerve tissue, including means for detecting and monitoring neuropathies in a mammal. The methods, devices and compositions include a morphogen or morphogen-stimulating agent provided to the mammal in a therapeutically effective concentration. In another embodiment, the invention provides methods and compositions which include a morphogen or morphogen-stimulating agent, and a nerve trophic factor or nerve trophic factor-stimulating agent at concentrations effective for stimulating dendrite outgrowth. The morphogen and the nerve trophic factor can be admixed in combination.
Owner:MARIEL THERAPEUTICS +1
Polymer-based sustained release device
ActiveUS20070166352A1Improve bioavailabilityMinimizes loss of activityPowder deliveryOrganic active ingredientsMedicineSugar
This invention relates to compositions for the sustained release of biologically active polypeptides, and methods of forming and using said compositions, for the sustained release of biologically active polypeptides. The sustained release compositions of this invention comprise a biocompatible polymer having dispersed therein, a biologically active polypeptide and a sugar.
Owner:ALKERMES PHARMA IRELAND LTD +1
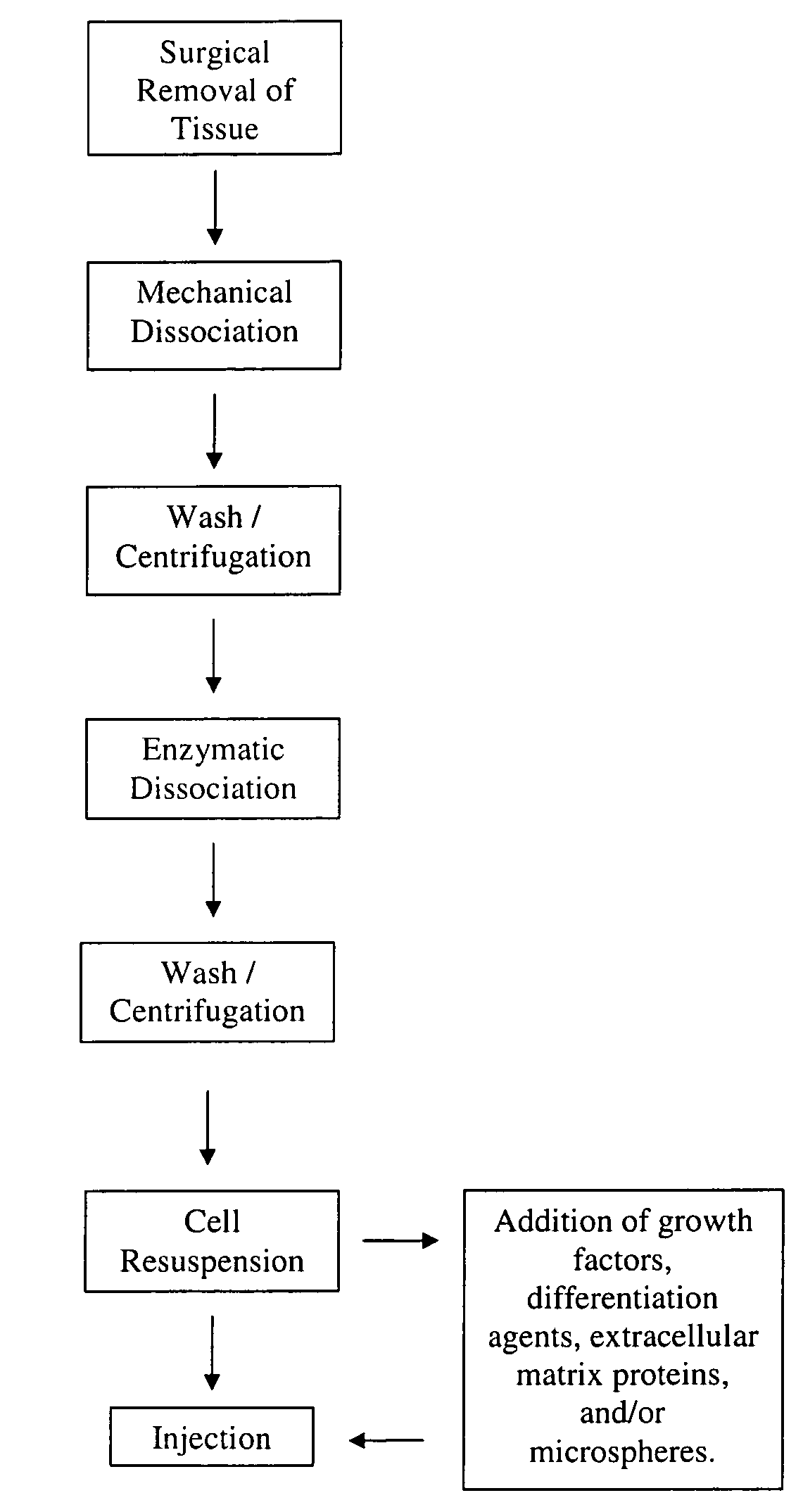
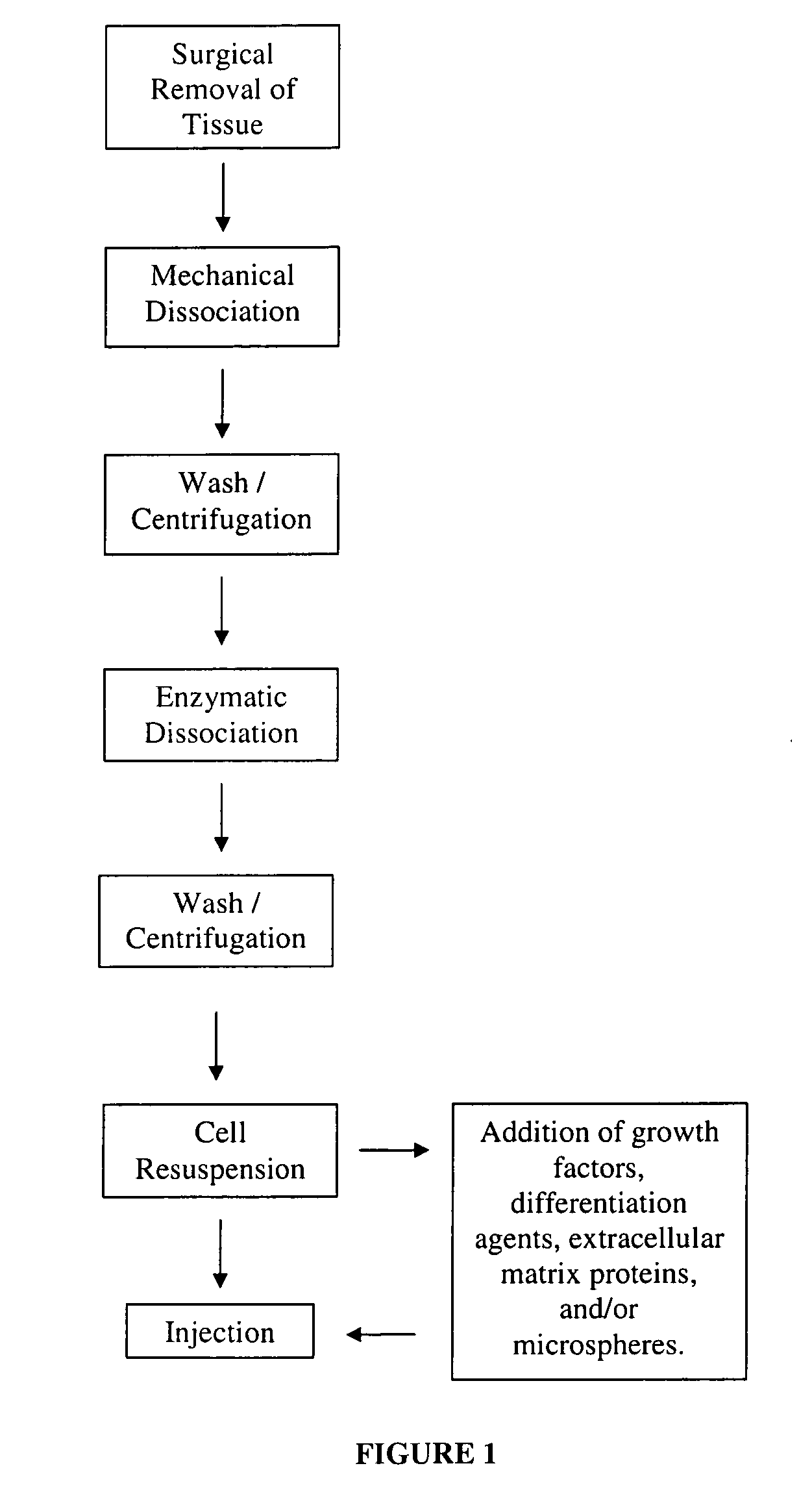
Nonexpansion Protocol for Autologous Cell-Based Therapies
InactiveUS20070224173A1Increased risk of contaminationIncrease the number of cellsBiocideOrganic active ingredientsHeterologousCell-Extracellular Matrix
The present application describes various applications of the non-expansion protocol for the preparation of an injectable autologous cell mixture of the present invention that can be used to prevent symptoms in a number of indications. Cells are isolated from surgically derived tissue and are at least partially disaggregated from each other. The heterologous cell mixture is mixed with growth factors, differentiation agents, extracellular matrix proteins and / or microspheres and injected into the patient without cell expansion. The harvesting of tissue, cell isolation, and injection are performed within a single surgical procedure lasting only minutes to hours.
Owner:BOSTON SCI SCIMED INC
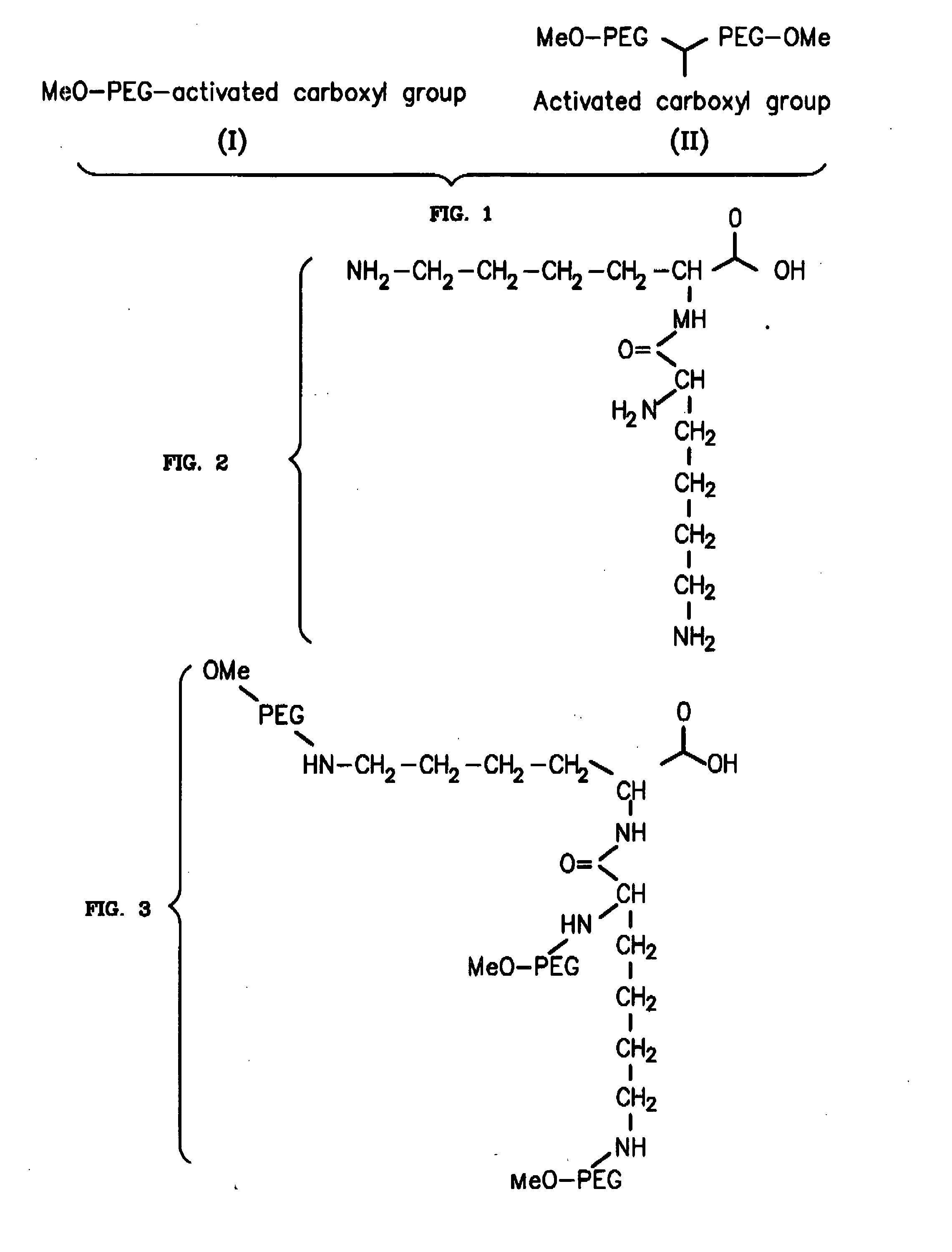
Novel PEGylation agent
InactiveUS20070184015A1Reduce compliancePromote degradationPeptide/protein ingredientsCalcitoninsHalf-lifeBlood plasma
To address the issue of degradation by enzymatic reactions to proteins and peptides, polyethylene glycol (PEGylation) of the proteins and peptides has been established. PEGylated proteins and peptides have increased plasma half-lives and reduced immunogenicity. To further improve and extend the plasma half-life of desired protein or peptide therapeutics, a novel branched molecule of PEG possessing three PEGs with a single point of attachment is designed in this invention disclosure.
Owner:HAHN SOONKAP
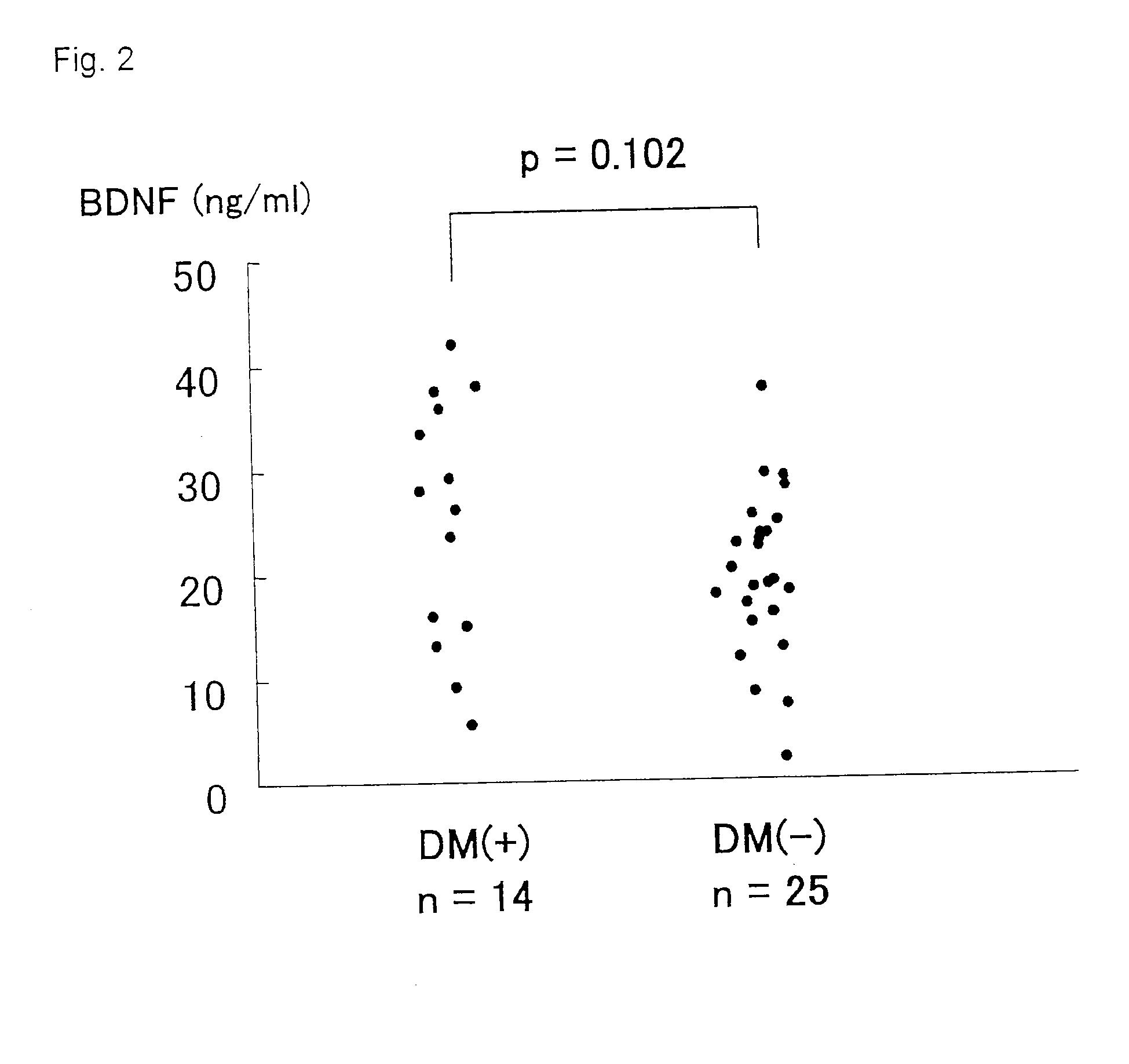
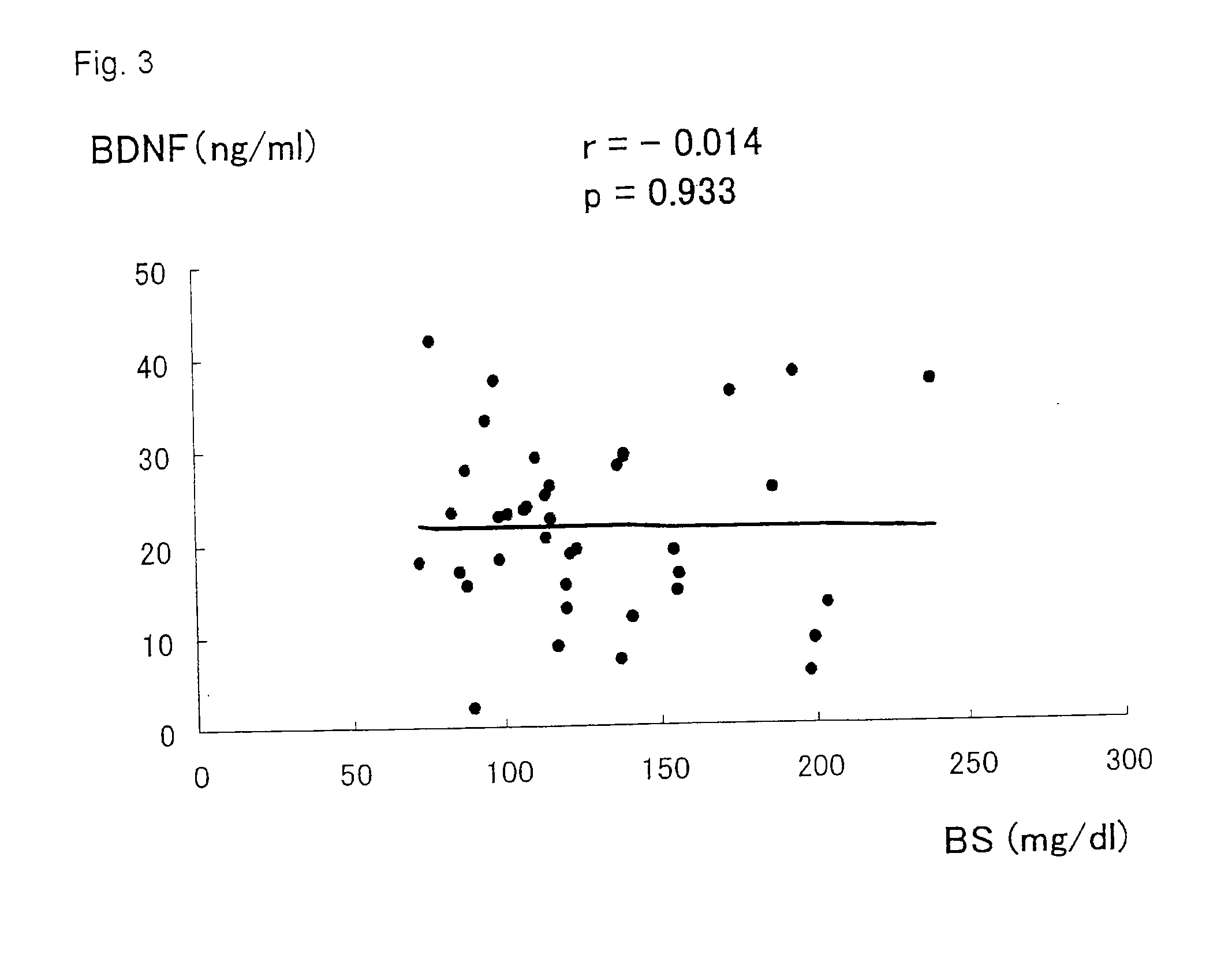
Diagnostic agent for ischemic heart disease risk group
InactiveUS20120122777A1Lower Level RequirementsSuppression problemBiocidePeptide/protein ingredientsDiagnostic agentAdditive ingredient
The present invention relates to a diagnostic agent for an ischemic heart disease risk group comprising an anti-brain-derived neurotrophic factor antibody as an effective ingredient, to an assay method for an ischemic heart disease risk group performed by measuring a brain-derived neurotrophic factor concentration in blood, and to a suppressive / preventive drug for ischemic heart disease, particularly for post-infarction myocardial remodeling, comprising a brain-derived neurotrophic factor.
Owner:MASAO DAIMON +1
Neuronal growth factor galectin-1
InactiveUS6890531B1Peptide/protein ingredientsAntibody mimetics/scaffoldsNerve degenerationADAMTS Proteins
This invention relates to a remedy for neuropathy, such as nerve injury, nerve degeneration, and hypofunction upon nerve grafting, which contains as the active ingredient galectin-1 having an amino acid sequence represented by SEQ ID NO:1 or its derivative; a protein having the amino acid sequence represented by SEQ ID NO:1 or one having a homology of 90% or more at the amino acid level with the sequence of SEQ ID NO:1 and carrying a disulfide bond(s) at least between Cys at the 16-position (Cys 16) and Cys at the 88-position (Cys 88) among cystein residues at the 2-position (Cys 2), 16-position (Cys 16), 42-position (Cys 42), 60-position (Cys 60), 88-position (Cys 88) and 130-position (Cys 130); and a process for producing the galectin-1 or its derivative protein by using an affinity column having an antibody to the above protein.
Owner:KIRIN BREWERY CO LTD
Rapid establishment and/or termination of substantial steady-state drug delivery
ActiveUS20110076317A1Rapid terminationLarge deliveryPeptide/protein ingredientsMedical devicesDiseaseSide effect
The present invention is directed to treatment methods for a disease or condition, in a subject in need of such treatment, that provide alternatives to treatment by injection that give, relative to treatment by injection, improved treatment outcomes, 100% treatment compliance, reduced side effects, and rapid establishment and / or termination of substantial steady-state drug delivery. The method typically includes providing continuous delivery of a drug from an implanted osmotic delivery device, wherein substantial steady-state delivery of the drug at therapeutic concentrations is typically achieved within about 7 days or less after implantation of the osmotic delivery device in the subject and the substantial steady-state delivery of the drug from the osmotic delivery device is continuous over a period of at least about 3 months. In one embodiment, the present invention is directed to treatment of type 2 diabetes mellitus using incretin mimetics.
Owner:INTARCIA THERAPEUTICS INC
Shaped microparticles for pulmonary drug delivery
InactiveUS20020128179A1Enhancing degradation characteristicPowder deliveryFactor VIICircular discMicrofabrication
Microparticles for use in the pulmonary delivery of a therapeutic material, comprising a polymer matrix, which is prefabricated to have a particular geometric shape including that of a disc cube, rectangle or snowflake. Additionally, these microparticles may include a winged structure to enhance the aerodynamic characteristics of said microparticle. Microfabrication methods for making these microparticles are provided.
Owner:BATTELLE MEMORIAL INST +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com