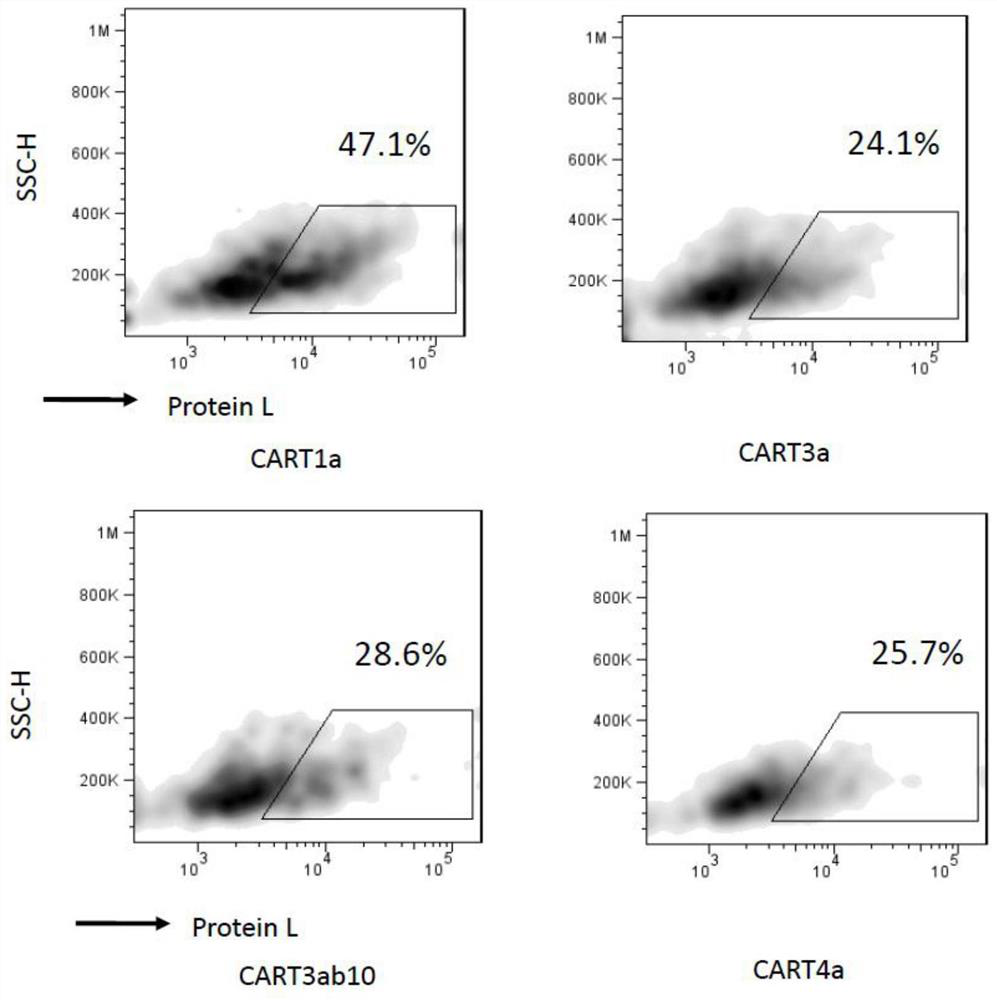
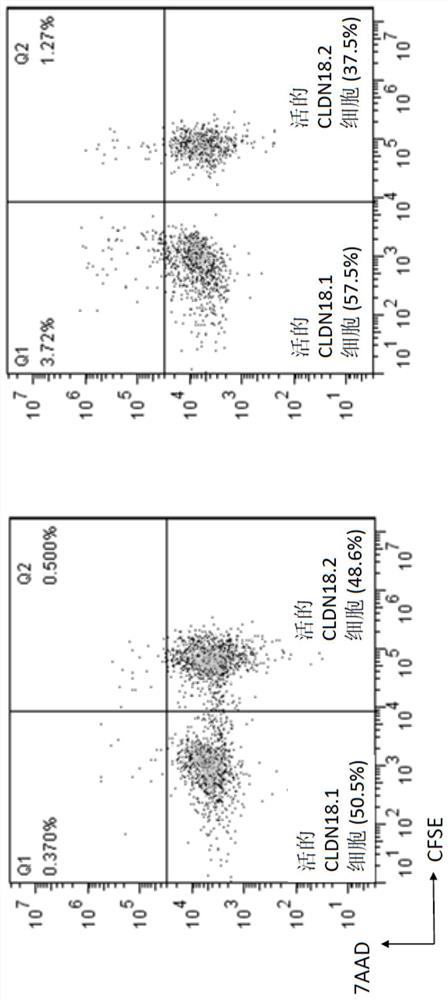
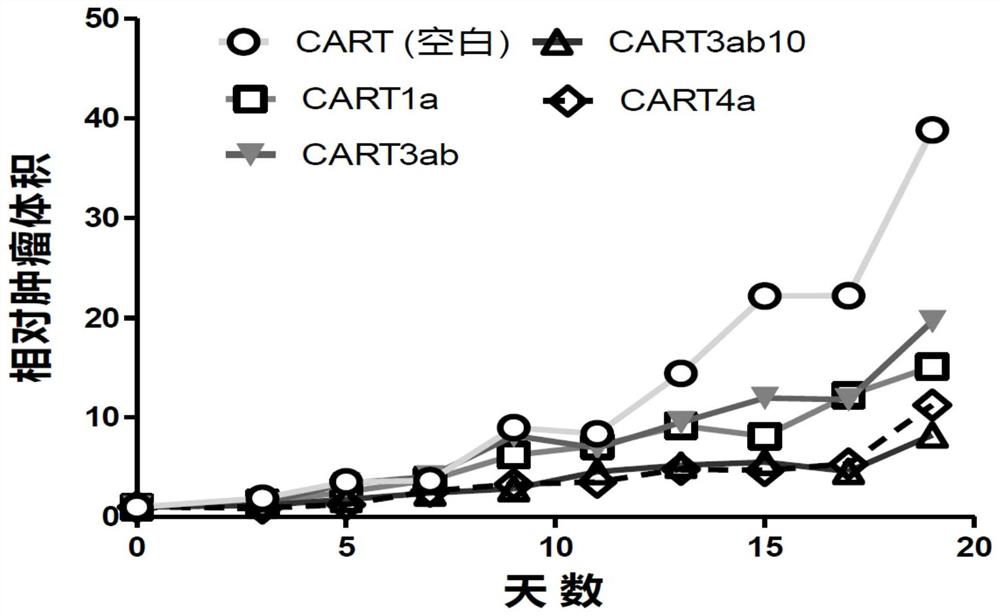
Claudin 18.2-targeting CAR molecule, immune cell modified by Claudin 18.2-targeting CAR molecule and application of Claudin 18.2-targeting CAR molecule
A nucleic acid construct and sequence technology, applied in the field of modified immune cells and CAR molecules, can solve the problems of poor immune cell effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0107] Cloning expression and purification of embodiment 1 recombinant protein, antibody
[0108] The recombinant proteins involved in the present invention, including the expression and purification of monoclonal antibodies, are obtained by cloning, expression and purification of the present invention. The vector used for expression cloning is pTT5 vector (Biovector, Cat#: 102762). All protein expressions (including antibody light and heavy chains) were purified after transiently transfecting HEK 293F cells (Life Technologies Cat. No. 11625019) with pTT5 vector.
[0109] 293F cells were expanded in Gibco FreeStyle 293Expression Medium (Gibco, Cat#12338018) medium. Before the start of the transient, adjust the cell concentration to 6-8×10 5 cell / ml, 1% FBS (Aus Gene X FBSExcellent supplier: AusGeneX, China, Cat#FBSSA 500-S), 37°C 8% CO 2 Cultured on a shaker for 24 hours, and the survival rate was >95% under the microscope again, and the cell concentration was 1.2×10 6 cel...
Embodiment 2
[0110] Example 2 Human CLDN18.2 binding ELISA experiment
[0111] The human CLDN18.2 high-expression cell line used in the present invention is completed through the company's stable cell line construction platform. Specific steps are as follows:
[0112] On the first day of the experiment, 293T cells (Cat#GNHu17, a cell bank of the Type Culture Collection Committee of the Chinese Academy of Sciences) were seeded in two 6cm culture dishes, and the number of cells in each culture dish reached 7.5×10 5 indivual. On the second day, the plasmids (pGag-pol, pVSV-G, pBabe, etc. BioVector, Plasmid Vector Strain Cell Gene Collection Center) and cloned human CLDN18.1 gene (NCBI Reference Sequence: NP_057453.1), human CLDN18.2 gene (NCBIReference Sequence: NP_001002026.1), mouse CLDN18.1 (NP_062789.1) or mouse CLDN18.2 (NP_001181850.1) gene plasmid pBabe each 4μg was added to OPTI-MEM (Thermofisher Scientific Cat#31985070), so that the final volume was 200 μl, prepare 200 μl OPTI-MEM...
Embodiment 3
[0114] Example 3 Discovery and effect identification of anti-human CLDN18.2 antibody
[0115] 1. Discovery of anti-human CLDN18.2 antibody
[0116] The anti-human CLDN18.2 monoclonal antibody of the present invention is a mouse immunized with the human CLDN18.2 high-expression cell line (hCLDN18.2+cell) obtained in Example 2, and the spleen of the immunized mouse is taken for hybridoma fusion, and obtained from several Screened and optimized from millions of hybridoma clones.
[0117] Experimental mice, female, 4 weeks old (SJL mice were purchased from Beijing Weitong Lihua Experimental Animal Technology Co., Ltd., animal production license number: SCXK (Beijing) 2016-0011; Balb / c mice were purchased from Shanghai West Poole-Bick Laboratory Animals Ltd). After purchase, they were raised in a laboratory environment for 1 week, with daytime light / night dark cycle adjustment, temperature 20-25°C, and humidity 40-60%. Mice were divided into 3 / group / cage.
[0118] Cultivate the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com