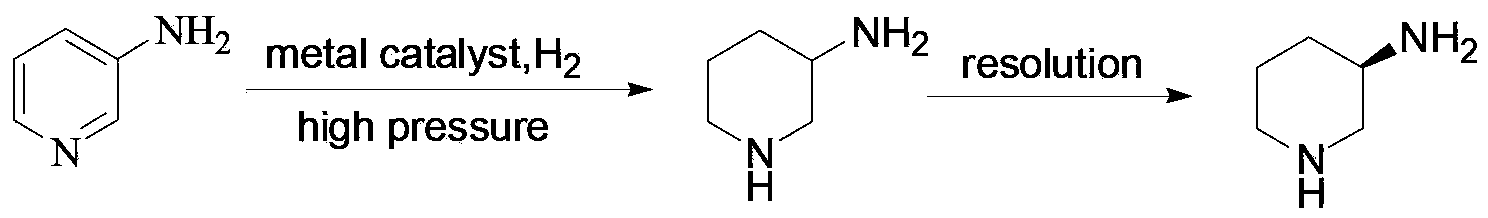
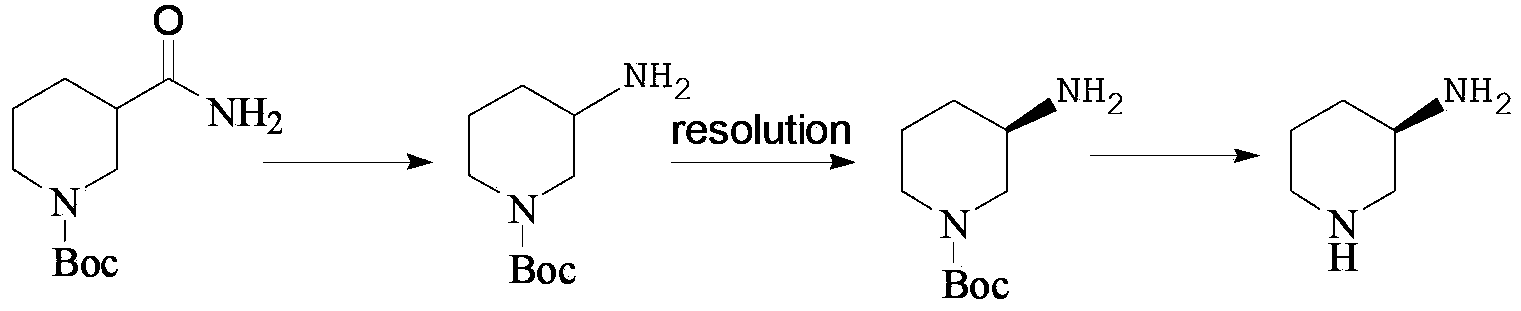
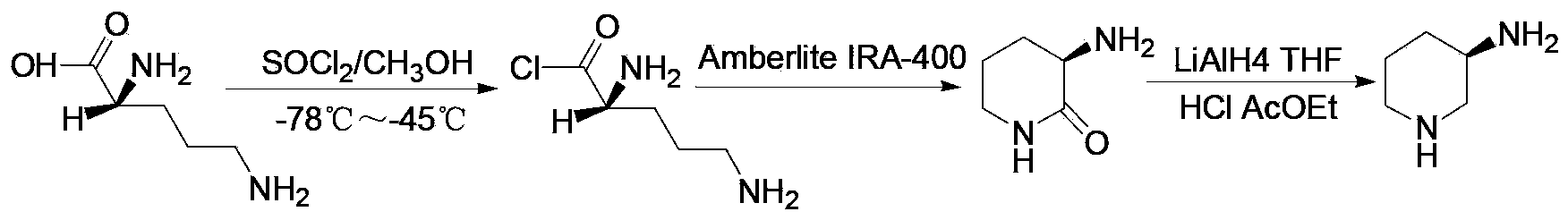
Preparation method of amino protection (R)-3-amino piperidine
A technology of amino protection and aminopiperidine is applied in the field of preparation of optically active intermediates, can solve problems such as long steps, unsuitable for industrialized production and the like, and achieves the effects of high yield, readily available raw materials, and concise process steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Example 1 Preparation of (R)-2-(tert-butoxycarbonyl) glutaric acid (a compound of formula III)
[0045]
[0046] 3-liter three-necked bottle, into which DMF / H 2O (volume ratio 3:2) mixed solvent 1.55 liters, while stirring, slowly add 142 grams of D-glutamic acid to the system, stir for 10 minutes until the system is clear, keep the system at 20°C to 30°C, add dicarbonic acid dropwise 161 grams of di-tert-butyl ester was stirred for 1 hour after dripping. Rotate distillation at about 55°C to remove most of the solvent, add 1 liter of ethyl acetate to it, separate the layers, wash the organic phase with 200 ml of 1 mole per liter of hydrochloric acid solution, extract the water phase with 200 ml of ethyl acetate, combine the organic phases, The organic phase was washed with 300 ml of water, and then washed with 300 ml of saturated brine, the organic phase was separated, dried with an appropriate amount of anhydrous sodium sulfate for 1 hour, filtered, and the filtrat...
Embodiment 2
[0047] Example 2 Preparation of (R)-2-(phthaloyl)aminoglutaric acid (a compound of formula III)
[0048]
[0049] 3-liter three-necked bottle, into which DMF / H 2 O (volume ratio 3:2) mixed solvent 1.55 liters, while stirring, slowly add 147 grams of D-glutamic acid to the system, stir for 10 minutes until the system is clear, keep the system at 15°C to 20°C, add o-benzene 178 grams of diformic anhydride, after dropping, stirred for 2 hours. Rotate distillation at about 55°C to remove most of the solvent, add 1 liter of ethyl acetate to it, separate the layers, wash the organic phase with 200 ml of 1 mole per liter of hydrochloric acid solution, extract the water phase with 200 ml of ethyl acetate, combine the organic phases, The organic phase was washed with 300 ml of water, and then washed with 300 ml of saturated brine, the organic phase was separated, dried with an appropriate amount of anhydrous sodium sulfate for 1 hour, filtered, and the filtrate was spin-dried to ob...
Embodiment 3
[0050] Example 3 Preparation of (R)-2-tert-butyloxycarbonylamino-1,5-pentanediol (a compound of formula IV)
[0051]
[0052] In a 5-liter three-necked flask, replace the system with nitrogen three times, add 2 liters of dry tetrahydrofuran to it, add 200 grams of (R)-2-(tert-butoxycarbonyl) glutaric acid prepared in Example 1 while stirring, and cool down to -5°C About 197 grams of borane dimethyl sulfide was added dropwise therein (the molar concentration was 10 moles per liter), keeping the temperature of the system not exceeding 0°C, rising to about 50°C after dropping, and stirring for 10 hours. Cool down to below 10°C, slowly add 200 ml of methanol dropwise, after the drop is completed, transfer the system to rotary distillation, spin dry, add 1 liter of ethyl acetate to the system, and use 0.5 moles per liter of sodium hydroxide aqueous solution 200 Wash the organic phase with 1 ml, then wash the organic phase with 200 ml of saturated brine, separate the organic phas...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com