Synthetic method and application of MACAmide
A technology of macamide and a synthesis method, which is applied in the synthesis of macamide and the field of macamide, can solve the problems of complex components of maca, low purity of macamide, limited source of raw materials, etc., and achieves the treatment of male sexual dysfunction. , Improve male fertility, the effect of easy control of operating conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
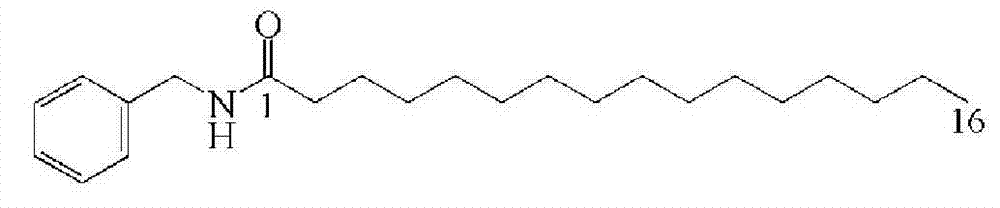
Embodiment 1
[0034] A kind of one-step synthetic macamide---the method of N-benzyl-hexadecanamide:
[0035]Weigh 0.01mol HOAt, 0.01mol EDC·HCl and 0.01mol DIPEA respectively in 7.3mL DCM (dichloromethane), then add 0.01mol benzylamine, 0.01mol palmitic acid, the ratio of reaction raw materials to DCM is 50% (w / v), stirring overnight at 10°C; adding 50mL of deionized water, stirring at room temperature for 30min, filtering and vacuum drying to obtain the product;
[0036] Performance Testing:
[0037] Infrared: FT / IR-660 infrared meter, the peak value is V max =3303(N-H), 2917, 2849, 1639(C=O), 1549(N-C), 1454(N-C=O), 730 and 696 (benzene ring) cm -1 ;
[0038] Mass Spectrum: Thermo's LCQ DECAXP Ion Hydrazine Mass Spectrum, m / z346.2;
[0039] NMR: Bruker AVANCE III600 NMR instrument 1 H spectrum (600MHz, CDCl 3 ), δ=2.20(t,J=7.65Hz,2H,H=2), δ=1.65(t,J=7.05Hz,2H,H=3), δ=1.26(s,br,24H,H= 4-15), δ=0.88(t,J=6.75Hz,3H,H=16), δ=4.435(d,J=7.65Hz,2H,H=1`), δ=7.29(m,5H, H=3`-7`), δ=5.73(s,1...
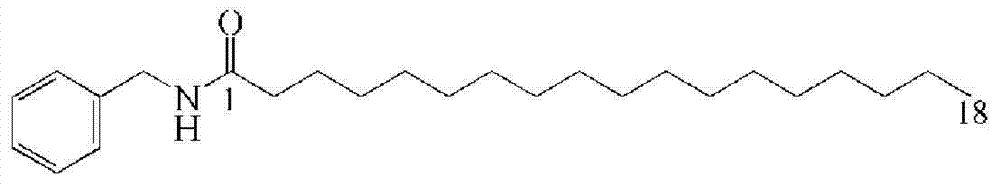
Embodiment 2
[0044] A kind of one-step chemical synthesis macamide---the method of N-benzyl-octadecylamide:
[0045] Weigh 0.1mol HOAt, 0.1mol EDC·HCl and 0.1mol DIPEA respectively in 2730mL DCM, then add 0.1mol benzylamine, 0.01mol stearic acid, the ratio of reaction materials to DCM is 0.5% (w / v) at 40°C Stir overnight under the same conditions, and evaporate the solvent to dryness; add 200 mL of deionized water, stir at room temperature for 30 min, filter and dry in vacuo to obtain the product;
[0046] Performance Testing:
[0047] Infrared: FT / IR-660 infrared meter, the peak value is V max =3306 (N-H), 2918, 2849, 1639 (C=O), 1552 (N-C), 1455 (N-C=O), 1428, 743, 730 and 699 (benzene ring) cm -1 ;
[0048] Mass Spectrum: Thermo's LCQ DECAXP Ion Hydrazine Mass Spectrum, m / z374.3;
[0049] NMR: Bruker AVANCE III600 NMR instrument 1 H spectrum (600MHz, CDCl 3 ), δ=2.20(t,J=7.65Hz,2H,H=2), δ=1.65(t,J=7.05Hz,2H,H=3), δ=1.29(s,br,24H,H= 4-17), δ=0.88(t,J=6.75Hz,3H,H=18), δ=4.44(d,J=7....
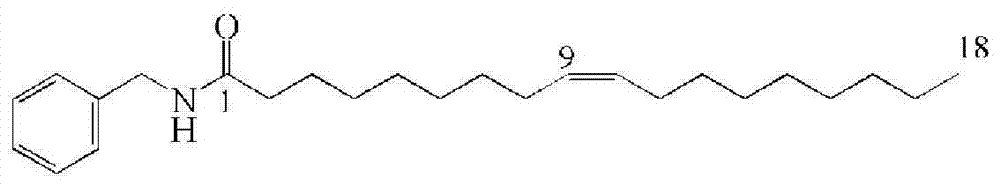
Embodiment 3
[0054] A method for chemically synthesizing macamide in one step——N-benzyl-9 cis-oleic acid amide:
[0055] Weigh 0.015mol HOAt, 0.02mol EDC·HCl and 0.03mol DIPEA into 30mL DCM, then add 0.01mol benzylamine and 0.01mol oleic acid, stir at 30°C for 12h; add 50mL of deionized water, stir at room temperature for 30min , filtered and dried in vacuo to obtain the product;
[0056] Performance Testing:
[0057] Infrared: FT / IR-660 infrared instrument, V max =3299 (N-H), 3002 (C=C-H), 2919, 2849, 1640 (C=O), 1554 (N-C), 1463.98 (N-C=O), 725 and 696 (benzene ring);
[0058] Mass Spectrum: Thermo's LCQ DECAXP Ion Hydrazine Mass Spectrum, m / z372.3;
[0059] NMR: Bruker AVANCE III600 NMR instrument 1 H spectrum (600MHz, CDCl 3 ), δ=2.21(t,J=7,65Hz,2H,H=2), δ=1.66(m,J=6,45Hz,2H,H=3), δ=1.28(s,br,8H, H=4-7), δ=2.047(m,J=3,90Hz,4H,H=8,11), δ=5.34(m,J=2,00Hz,2H,H=9,10), δ =1.30(s,br,12H,H=12-17), δ=0.879(t,J=6,60Hz,3H,H=18), δ=4,13(q,J=7,10Hz,2H ,H=1`), δ=7,319(m,5H,H=3`-7`), δ=5,65(...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com