A kind of method of synthesizing butenafine
A technology of butenafine and its compound, which is applied in the field of pharmaceutical chemical synthesis, can solve the problems of high synthesis cost, complicated operation, and easy oxidation, and achieve the effects of simple synthesis method, wide source of raw materials, and avoidance of residues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] (1) The compound of formula I, i.e. the preparation of N-(4-(tert-butyl)benzyl)-1-naphthylcarboxamide
[0062] Add 20 mL of CH to a 100 mL round bottom flask 2 Cl 2 and 5mmol 4-tert-butylbenzylamine, dissolved. Then 20 mmol of triethylamine was added, and the mixture was stirred at 20° C. (using an IKA magnetic stirrer, RCT basic type, stirring speed 500 rpm). 7.5 mmol of 1-naphthoyl chloride was slowly added dropwise. After reacting for 10 h, add 30 mL of saturated NaHCO 3 solution, extracted with ether (20mL x 3), the organic phase was dried with anhydrous sodium sulfate, filtered, the organic phase was concentrated by a rotary evaporator (Buchi Co., Ltd., Switzerland, BUCHI rotary evaporator R-3), and then passed through a chromatography column ( Beijing Xinweier Glass Instrument Co., Ltd., C383040C chromatography column with sand plate storage ball, 35 / 20, φ30mm, effective length: 500ml) was separated by chromatography to obtain light yellow crystal product N-(4...
Embodiment 2
[0069] (1) The compound of formula I, i.e. the preparation of N-(4-(tert-butyl)benzyl)-1-naphthylcarboxamide
[0070] Carry out with the procedure same as Example 1, just change 5mmol 1-naphthoyl chloride into 5.0mmol 1-naphthoyl chloride, after reacting at 20 ℃ for 10h, obtain light yellow crystal product N-( 4-(tert-butyl)benzyl)-1-naphthylcarboxamide, yield 75%.
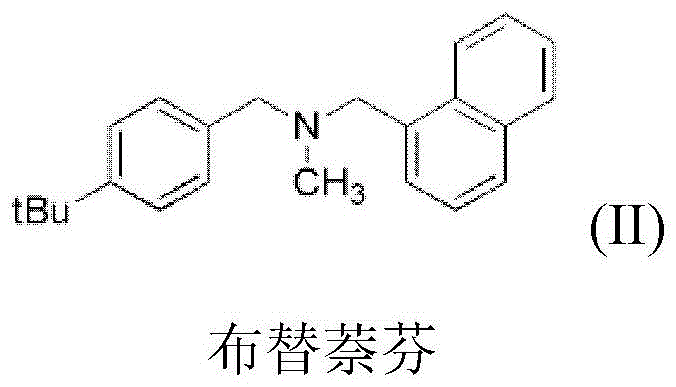
[0071] (2) the target product of formula II, i.e. the preparation of N-methyl-N-(naphthalene-1-ylmethyl)-1-(4-tert-butylphenyl) methylamine
[0072] The same procedure as in Example 1 was followed, except that the reaction mixture was heated at 100° C. for 18 h and then cooled to room temperature. After the same post-treatment, a white crystal product was isolated with a yield of 85%.
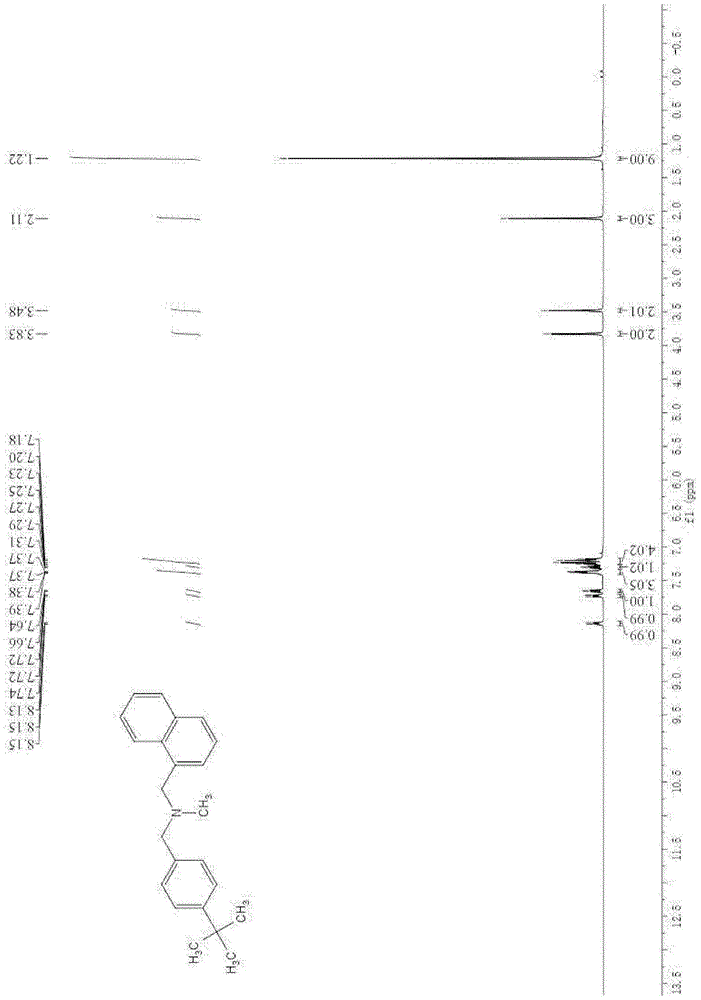
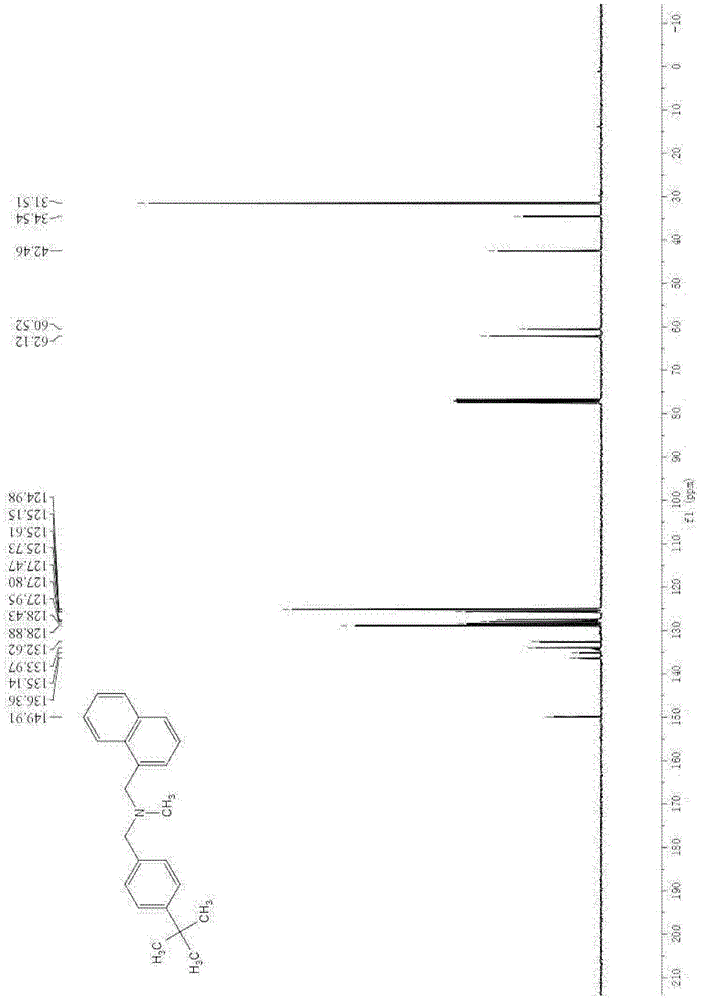
[0073] Gained product is carried out proton nuclear magnetic resonance spectrum and carbon nuclear magnetic resonance spectrum analysis and confirms that gained product is N-methyl-N-(naphthalene-1-ylmethyl)-1-(4-tert-butylphenyl...
Embodiment 3
[0075] (1) The compound of formula I, i.e. the preparation of N-(4-(tert-butyl)benzyl)-1-naphthylcarboxamide
[0076] With embodiment 1.
[0077] (2) the target product of formula II, i.e. the preparation of N-methyl-N-(naphthalene-1-ylmethyl)-1-(4-tert-butylphenyl) methylamine
[0078] The same procedure as in Example 1 was carried out except that the reaction mixture was heated at 120° C. for 20 h and then cooled to room temperature. After the same post-treatment, a white crystal product was isolated with a yield of 92%.
[0079] Gained product is carried out proton nuclear magnetic resonance spectrum and carbon nuclear magnetic resonance spectrum analysis, confirms that gained product is N-methyl-N-(naphthalene-1-ylmethyl)-1-(4-tert-butylphenyl) methylamine, That is butenafine.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com