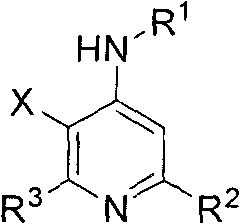
Multi-substituted fluorine-containing pyridine
A multi-substituted, pyridine technology, applied in the multi-substituted pyridine and synthesis fields, can solve the problems of difficulty in introducing fluorine atoms and not many fluorine-containing pyridines.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
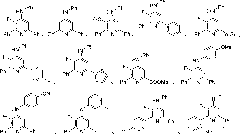
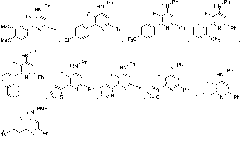
Image
Examples
Embodiment 1
[0051] Experimental procedure
[0052] Add alkynyl imine 1 (0.4 mmol) into a solution of tetrahydrofuran (2 mL) dissolved with 3 equivalents of methylamine 2 and 2.5 equivalents of cesium carbonate at 80°C for 2-7 hours (24 hours for 1e). Monitor the response. After the reaction, the reaction solution was directly filtered, the filtrate was spin-dried, and the residue was subjected to column chromatography to obtain product 3.
[0053] Compound data
[0054] 1.3-fluoro-N,2,6-triphenylpyridin-4-amine
[0055] 3-fluoro-4-phenylamino-2,6-diphenylpyridine (3a)
[0056]
[0057] White solid, melting point 127-129°C; 1 H NMR (300MHz, CDCl 3 )δ8.02(d, J=7.1Hz, 2H), 7.95(d, J=7.6Hz, 2H), 7.56-7.14(m, 12H), 6.34(d, J=3.3Hz, 1H); 19 F NMR (282MHz, CDCl 3 )δ-152.37; 13 C NMR (100MHz, CDCl 3 )δ153.3(d, J=5.9Hz), 146.9(d, J=253.1Hz), 144.0(d, J=8.1Hz), 140.6(d, J=10.2Hz), 139.5, 139.2, 136.0(d , J=5.1Hz), 129.8, 129.0, 129.0 (d, J=5.9Hz), 128.7, 128.6, 128.4, 127.0, 124.7, 122...
Embodiment 2
[0139] Experimental procedure
[0140] The alkynyl imine 1 (0.4 mmol) was added into a tetrahydrofuran solution (2 mL) dissolved with 2.0 equivalent of n-butylamine to react at 80°C for 2 hours, and the reaction was monitored by thin-layer chromatography. After the reaction, the reaction solution was directly spin-dried, and then the intermediate 4v was obtained through column chromatography. Dissolve it in 2 ml of anhydrous tetrahydrofuran, add sodium hydride at room temperature, then slowly raise the temperature to 80°C, react for about 20 minutes, quench with ice water, then separate, dry, and column chromatography to obtain Product 3v.
[0141] Compound data
[0142] 22.3-fluoro-N, 6-diphenyl-2-propylpyridin-4-amine
[0143] 3-Fluoro-4-phenylamino-6-phenyl-2-propylpyridine (3v)
[0144]
[0145] yellow liquid; 1 H NMR (300MHz, CDCl 3 )δ7.81 (d, J=2.9Hz, 2H), 7.43-7.03 (m, 9H), 6.23 (s, 1H), 2.90-2.79 (m, 2H), 1.92-1.75 (m, 2H), 1.04 (t, J=7.4Hz, 3H); 19 F NMR (2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com