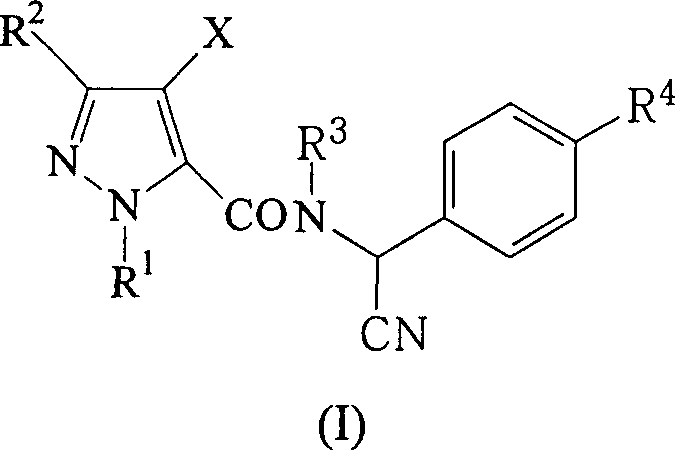
Alpha-cyano-N-benzylpyrazoamide compound, preparation method and pest control agent thereof as active component
A technology of benzyl pyrazole amide and compound, applied in the field of pest control agents, can solve the problems such as the application of α-cyano-N-benzyl pyrazole amide compounds that are not reported in the literature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
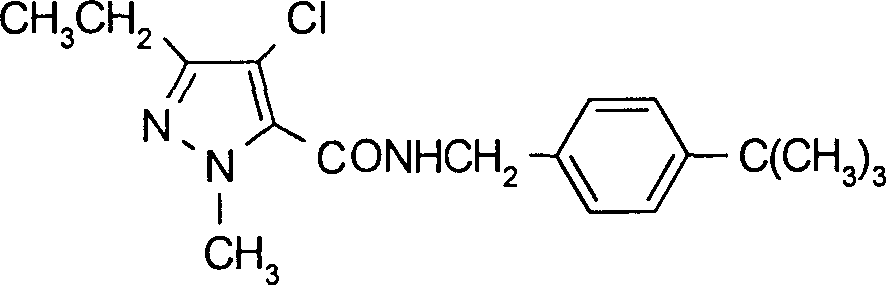
[0060] Example 1: Preparation of N-(α-cyano-4-tert-butylbenzyl)-4-chloro-3-ethyl-1-methylpyrazole-5-carboxamide
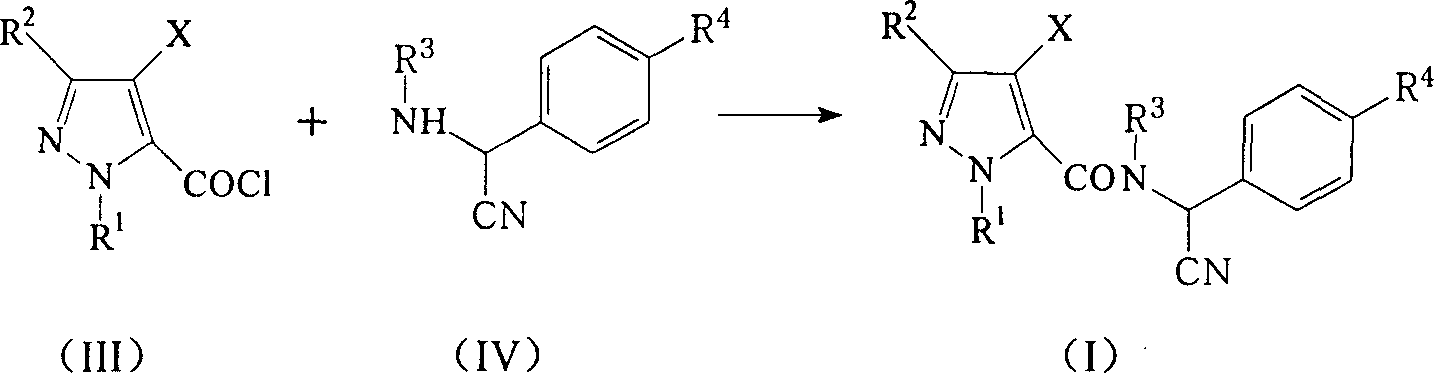
[0061] A solution composed of freshly prepared 4-chloro-3-ethyl-1-methylpyrazole-5-formyl chloride (12.0g, 0.055mol) and toluene (30ml) was slowly added dropwise under stirring and ice-water bath cooling To a mixture consisting of α-cyano-4-tert-butylbenzylamine (10.0g, 0.05mol), triethylamine (6.0g, 0.06mol) and toluene (200ml). After the dropwise addition, the temperature of the reaction mixture was raised to about 30° C., and the stirring was continued at this temperature. It took about 4-5 hours to follow the chromatographic tracking until the reactant was basically converted completely. The resulting reaction mixture was poured into water (300ml), and the layers were separated. The organic layer was washed with saturated brine, dried over anhydrous sodium sulfate, and precipitated under reduced pressure. The residue was the title compound (Compound No.6 in Ta...
Embodiment 2
[0063] According to the method described in Example 1, other α-cyano-N-benzylpyrazole amide compounds described in Table 1 were synthesized using the corresponding pyrazole carboxyl chloride and α-cyanobenzylamine or its salt.
[0064]
[0065] compound
[0066] 11
[0067] Formulation examples using the compound of the present invention as an active ingredient, which are useful as insecticides, acaricides or fungicides in the fields of agriculture, horticulture and floriculture, will be described below. However, embodiments of the present invention are not limited to the following.
preparation Embodiment 1
[0068] Formulation Example 1: wettable powder
[0069] 20 parts of the compound of the present invention, 10 parts of white carbon black, 55 parts of kaolin, 10 parts of sodium lauryl sulfate and 5 parts of calcium lignosulfonate were uniformly mixed together and jet milled to obtain a wettable powder with an active ingredient of 20%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com